Embryological features of Tofieldia glutinosa and their bearing on the early diversification of monocotyledonous plants
- PMID: 18511412
- PMCID: PMC2712362
- DOI: 10.1093/aob/mcn084
Embryological features of Tofieldia glutinosa and their bearing on the early diversification of monocotyledonous plants
Abstract
Background and aims: Although much is known about the vegetative traits associated with early monocot evolution, less is known about the reproductive features of early monocotyledonous lineages. A study was made of the embryology of Tofieldia glutinosa, a member of an early divergent monocot clade (Tofieldiaceae), and aspects of its development were compared with the development of other early divergent monocots in order to gain insight into defining reproductive features of early monocots.
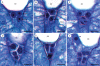
Methods: Field-collected developing gynoecial tissues of Tofieldia glutinosa were prepared for histological examination. Over 600 ovules were sectioned and studied using brightfield, differential interference contrast, and fluorescence microscopy. High-resolution digital imaging was used to document important stages of megasporogenesis, megagametogenesis and early endosperm development.
Key results: Development of the female gametophyte in T. glutinosa is of a modified Polygonum-type. At maturity the female gametophyte is seven-celled and 11-nucleate with a standard three-celled egg apparatus, a binucleate central cell (where ultimately, the two polar nuclei will fuse into a diploid secondary nucleus) and three binucleate antipodal cells. The antipodal nuclei persist past fertilization, and the process of double fertilization appears to yield a diploid zygote and triploid primary endosperm cell, as is characteristic of plants with Polygonum-type female gametophytes. Endosperm development is helobial, and free-nuclear growth initially proceeds at equal rates in both the micropylar and chalazal endosperm chambers.
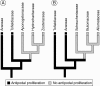
Conclusions: The analysis suggests that the shared common ancestor of monocots possessed persistent and proliferating antipodals similar to those found in T. glutinosa and other early-divergent monocots (e.g. Acorus and members of the Araceae). Helobial endosperm among monocots evolved once in the common ancestor of all monocots excluding Acorus. Thus, the analysis further suggests that helobial endosperm in monocots is homoplasious with those helobial endosperms that are present in water lilies and eudicot angiosperms.
Figures












Similar articles
-
Endosperm development in the Araceae (Alismatales) and evolution of developmental modes in monocots.J Plant Res. 2010 Nov;123(6):731-9. doi: 10.1007/s10265-010-0327-4. Epub 2010 Apr 6. J Plant Res. 2010. PMID: 20364441
-
Modularity of the angiosperm female gametophyte and its bearing on the early evolution of endosperm in flowering plants.Evolution. 2003 Feb;57(2):216-30. doi: 10.1111/j.0014-3820.2003.tb00257.x. Evolution. 2003. PMID: 12683519
-
The four-celled female gametophyte of Illicium (Illiciaceae; Austrobaileyales): implications for understanding the origin and early evolution of monocots, eumagnoliids,and eudicots.Am J Bot. 2004 Mar;91(3):332-51. doi: 10.3732/ajb.91.3.332. Am J Bot. 2004. PMID: 21653390
-
Comparative embryology of basal angiosperms.Curr Opin Plant Biol. 2001 Feb;4(1):14-20. doi: 10.1016/s1369-5266(00)00129-1. Curr Opin Plant Biol. 2001. PMID: 11163162 Review.
-
Developmental and evolutionary hypotheses for the origin of double fertilization and endosperm.C R Acad Sci III. 2001 Jun;324(6):559-67. doi: 10.1016/s0764-4469(01)01326-9. C R Acad Sci III. 2001. PMID: 11455879 Review.
Cited by
-
The gametic central cell of Arabidopsis determines the lifespan of adjacent accessory cells.Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22350-5. doi: 10.1073/pnas.1012795108. Epub 2010 Dec 6. Proc Natl Acad Sci U S A. 2010. PMID: 21135240 Free PMC article.
-
Endosperm development in the Araceae (Alismatales) and evolution of developmental modes in monocots.J Plant Res. 2010 Nov;123(6):731-9. doi: 10.1007/s10265-010-0327-4. Epub 2010 Apr 6. J Plant Res. 2010. PMID: 20364441
-
An F-Actin Mega-Cable Is Associated With the Migration of the Sperm Nucleus During the Fertilization of the Polarity-Inverted Central Cell of Agave inaequidens.Front Plant Sci. 2021 Nov 24;12:774098. doi: 10.3389/fpls.2021.774098. eCollection 2021. Front Plant Sci. 2021. PMID: 34899803 Free PMC article.
-
Embryogenesis in Polianthes tuberosa L var. Simple: from megasporogenesis to early embryo development.Springerplus. 2016 Oct 18;5(1):1804. doi: 10.1186/s40064-016-3528-z. eCollection 2016. Springerplus. 2016. PMID: 27812444 Free PMC article.
-
Embryo sac formation and early embryo development in Agave tequilana (Asparagaceae).Springerplus. 2014 Oct 1;3:575. doi: 10.1186/2193-1801-3-575. eCollection 2014. Springerplus. 2014. PMID: 25332875 Free PMC article.
References
-
- Anton AM, Cocucci AE. The grass megagametophyte and its possible phylogenetic implications. Plant Sytematics and Evolution. 1984;146:117–121.
-
- APG II. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society. 2003;141:399–436.
-
- Arber A. Monocotyledons: a morphological study. Cambridge: Cambridge University Press; 1925.
-
- Asplund I. Embryological studies in the genus Typha. Svensk Botanisk Tidskrift. 1972;66:1–17.
-
- Asplund I. Embryological studies in the genus Sparganium. Svensk Botanisk Tidskrift. 1973;67:177–200.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

