Novel variants in human Aquaporin-4 reduce cellular water permeability
- PMID: 18511455
- PMCID: PMC2733814
- DOI: 10.1093/hmg/ddn138
Novel variants in human Aquaporin-4 reduce cellular water permeability
Abstract
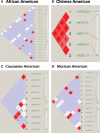
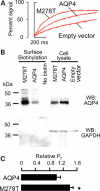
Cerebral edema contributes significantly to morbidity and mortality after brain injury and stroke. Aquaporin-4 (AQP4), a water channel expressed in astrocytes, plays a key role in brain water homeostasis. Genetic variants in other aquaporin family members have been associated with disease phenotypes. However, in human AQP4, only one non-synonymous single-nucleotide polymorphism (nsSNP) has been reported, with no characterization of protein function or disease phenotype. We analyzed DNA from an ethnically diverse cohort of 188 individuals to identify novel AQP4 variants. AQP4 variants were constructed by site-directed mutagenesis and expressed in cells. Water permeability assays in the cells were used to measure protein function. We identified 24 variants in AQP4 including four novel nsSNPs (I128T, D184E, I205L and M224T). We did not observe the previously documented M278T in our sample. The nsSNPs found were rare ( approximately 1-2% allele frequency) and heterozygous. Computational analysis predicted reduced function mutations. Protein expression and membrane localization were similar for reference AQP4 and the five AQP4 mutants. Cellular assays confirmed that four variant AQP4 channels reduced normalized water permeability to between 26 and 48% of the reference (P < 0.001), while the M278T mutation increased normalized water permeability (P < 0.001). We identified multiple novel AQP4 SNPs and showed that four nsSNPs reduced water permeability. The previously reported M278T mutation resulted in gain of function. Our experiments provide insight into the function of the AQP4 protein. These nsSNPs may have clinical implications for patients with cerebral edema and related disorders.
Figures




References
-
- Manley G.T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A.W., Chan P., Verkman A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. - PubMed
-
- Hiroaki Y., Tani K., Kamegawa A., Gyobu N., Nishikawa K., Suzuki H., Walz T., Sasaki S., Mitsuoka K., Kimura K., Mizoguchi A., Fujiyoshi Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol. 2006;355:628–639. - PubMed
-
- Jung J.S., Preston G.M., Smith B.L., Guggino W.B., Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. 1994;269:14648–14654. - PubMed
-
- Walz T., Hirai T., Murata K., Heymann J.B., Mitsuoka K. The three-dimensional structure of aquaporin-1. Nature. 1997;387:624–627. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

