Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage
- PMID: 18511517
- PMCID: PMC2438294
- DOI: 10.2353/ajpath.2008.070494
Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage
Abstract
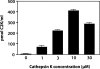
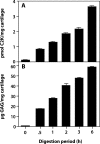
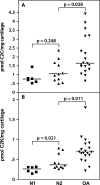
Cathepsin K is a cysteine protease of the papain family that cleaves triple-helical type II collagen, the major structural component of the extracellular matrix of articular cartilage. In osteoarthritis (OA), the anabolic/catabolic balance of articular cartilage is disrupted with the excessive cleavage of collagen II by collagenases or matrix metalloproteinases. A polyclonal antibody against a C-terminal neoepitope (C2K) generated in triple-helical type II collagen by the proteolytic action of cathepsin K was prepared and used to develop an enzyme-linked immunosorbent assay to study the generation of this epitope and the effects of its presence in normal adult and osteoarthritic femoral condylar articular cartilage. The generation of the C2K epitope in explant culture and the effect of a specific cathepsin K inhibitor were studied. The neoepitope, which is not generated by the collagenase matrix metalloproteinase-13, increased with age in articular cartilage and was significantly elevated in osteoarthritic cartilage compared with adult nonarthritic cartilage. Moreover, in explants from three of eight OA patients, the generation of the neoepitope in culture was significantly reduced by a specific, nontoxic inhibitor of cathepsin K. These data suggest that cathepsin K is involved in the cleavage of type II collagen in human articular cartilage in certain OA patients and that it may play a role in both OA pathophysiology and the aging process.
Figures






References
-
- Dahlberg L, Billinghurst RC, Manner P, Nelson F, Webb G, Ionescu M, Reiner A, Tanzer M, Zukor D, Chen J, Van Wart HE, Poole AR. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum. 2000;43:673–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

