Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses
- PMID: 18511559
- PMCID: PMC2402387
- DOI: 10.1073/pnas.0802866105
Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses
Erratum in
- Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):11032
Abstract
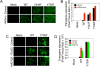
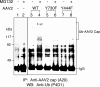
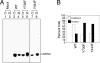
Recombinant adeno-associated virus 2 (AAV2) vectors are in use in several Phase I/II clinical trials, but relatively large vector doses are needed to achieve therapeutic benefits. Large vector doses also trigger an immune response as a significant fraction of the vectors fails to traffic efficiently to the nucleus and is targeted for degradation by the host cell proteasome machinery. We have reported that epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK) signaling negatively affects transduction by AAV2 vectors by impairing nuclear transport of the vectors. We have also observed that EGFR-PTK can phosphorylate AAV2 capsids at tyrosine residues. Tyrosine-phosphorylated AAV2 vectors enter cells efficiently but fail to transduce effectively, in part because of ubiquitination of AAV capsids followed by proteasome-mediated degradation. We reasoned that mutations of the surface-exposed tyrosine residues might allow the vectors to evade phosphorylation and subsequent ubiquitination and, thus, prevent proteasome-mediated degradation. Here, we document that site-directed mutagenesis of surface-exposed tyrosine residues leads to production of vectors that transduce HeLa cells approximately 10-fold more efficiently in vitro and murine hepatocytes nearly 30-fold more efficiently in vivo at a log lower vector dose. Therapeutic levels of human Factor IX (F.IX) are also produced at an approximately 10-fold reduced vector dose. The increased transduction efficiency of tyrosine-mutant vectors is due to lack of capsid ubiquitination and improved intracellular trafficking to the nucleus. These studies have led to the development of AAV vectors that are capable of high-efficiency transduction at lower doses, which has important implications in their use in human gene therapy.
Conflict of interest statement
Conflict of interest statement: The Sponsor declares a conflict of interest (such as defined by PNAS policy). K.I.B. is on the Scientific Advisory Board of Applied Genetic Technologies Corporation. The authors declare a conflict of interest (such as defined by PNAS policy). N.M. is an inventor of patents related to recombinant AAV technology and owns equity in a gene therapy company that is commercializing AAV for gene therapy applications.
Figures



References
-
- Berns KI, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. - PubMed
-
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. - PubMed
-
- Snyder RO, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB-002073/EB/NIBIB NIH HHS/United States
- R01 DK-062302/DK/NIDDK NIH HHS/United States
- R01 EB002073/EB/NIBIB NIH HHS/United States
- R01 GM082946/GM/NIGMS NIH HHS/United States
- R01 HL-07691/HL/NHLBI NIH HHS/United States
- R01 HL065570/HL/NHLBI NIH HHS/United States
- P01 HL-078810/HL/NHLBI NIH HHS/United States
- P01 HL059412/HL/NHLBI NIH HHS/United States
- P01 HL051811/HL/NHLBI NIH HHS/United States
- P01 HL078810/HL/NHLBI NIH HHS/United States
- R01 DK062302/DK/NIDDK NIH HHS/United States
- P01 HL-51811/HL/NHLBI NIH HHS/United States
- R01 AI051390/AI/NIAID NIH HHS/United States
- P01 DK058327/DK/NIDDK NIH HHS/United States
- R01 GM-082946/GM/NIGMS NIH HHS/United States
- P01 DK 058327/DK/NIDDK NIH HHS/United States
- R01 HL-65570/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

