Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers
- PMID: 18511600
- PMCID: PMC3633413
- DOI: 10.1634/stemcells.2008-0115
Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers
Abstract
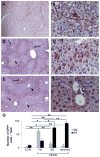
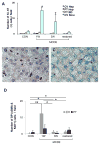
Liver injury activates quiescent hepatic stellate cells (Q-HSC) to proliferative myofibroblasts. Accumulation of myofibroblastic hepatic stellate cells (MF-HSC) sometimes causes cirrhosis and liver failure. However, MF-HSC also promote liver regeneration by producing growth factors for oval cells, bipotent progenitors of hepatocytes and cholangiocytes. Genes that are expressed by primary hepatic stellate cell (HSC) isolates overlap those expressed by oval cells, and hepatocytic and ductular cells emerge when HSC are cultured under certain conditions. We evaluated the hypothesis that HSC are a type of oval cell and, thus, capable of generating hepatocytes to regenerate injured livers. Because Q-HSC express glial fibrillary acidic protein (GFAP), we crossed mice in which GFAP promoter elements regulated Cre-recombinase with ROSA-loxP-stop-loxP-green fluorescent protein (GFP) mice to generate GFAP-Cre/GFP double-transgenic mice. These mice were fed methionine choline-deficient, ethionine-supplemented diets to activate and expand HSC and oval cell populations. GFP(+) progeny of GFAP-expressing precursors were characterized by immunohistochemistry. Basal expression of mesenchymal markers was negligible in GFAP(+)Q-HSC. When activated by liver injury or culture, HSC downregulated expression of GFAP but remained GFP(+); they became highly proliferative and began to coexpress markers of mesenchyme and oval cells. These transitional cells disappeared as GFP-expressing hepatocytes emerged, began to express albumin, and eventually repopulated large areas of the hepatic parenchyma. Ductular cells also expressed GFAP and GFP, but their proliferative activity did not increase in this model. These findings suggest that HSC are a type of oval cell that transitions through a mesenchymal phase before differentiating into hepatocytes during liver regeneration. Disclosure of potential conflicts of interest is found at the end of this article.
Figures





References
-
- Kordes C, Sawitza I, Muller-Marbach A, et al. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–7. - PubMed
-
- Niki T, Pekny M, Hellemans K, et al. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–7. - PubMed
-
- Fujio K, Evarts RP, Hu Z, et al. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

