Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells
- PMID: 18511601
- PMCID: PMC3017477
- DOI: 10.1634/stemcells.2007-1104
Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells
Abstract
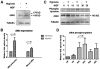
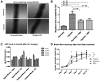
Mesenchymal stem cells (MSC) are adult multipotent cells found in bone marrow, adipose tissue, and other adult tissues. MSC have been shown to improve regeneration of injured tissues in vivo, but the mechanisms remain unclear. Typically, MSC are cultured under ambient, or normoxic, conditions (21% oxygen). However, the physiological niches for MSC in the bone marrow and other sites have much lower oxygen tension. When used as a therapeutic tool to repair tissue injuries, MSC cultured in standard conditions must adapt from 21% oxygen in culture to less than 1% oxygen in the ischemic tissue. We therefore examined the effects of preculturing human bone marrow-derived MSC in hypoxic conditions (1%-3% oxygen) to elucidate the best conditions that enhance their tissue regenerative potential. We demonstrated that MSC cultured in hypoxia activate the Akt signaling pathway while maintaining their viability and cell cycle rates. We also showed that MSC cultured in hypoxia induced expression of cMet, the major receptor for hepatocyte growth factor (HGF), and enhanced cMet signaling. MSC cultured in hypoxic conditions increased their migration rates. Since migration and HGF responsiveness are thought to be key mediators of MSC recruitment and/or activation in vivo, we next examined the tissue regenerative potential of MSC cultured under hypoxic conditions, using a murine hind limb ischemia model. We showed that local expression of HGF is increased in ischemic muscle in this model. Intra-arterial injection of MSC cultured in either normoxic or hypoxic conditions 24 hours after surgical induction of hind limb ischemia enhanced revascularization compared with saline controls. However, restoration of blood flow was observed significantly earlier in mice that had been injected with hypoxic preconditioned MSC. Collectively, these data suggest that preculturing MSC under hypoxic conditions prior to transplantation improves their tissue regenerative potential. Disclosure of potential conflicts of interest is found at the end of this article.
Conflict of interest statement
Figures




References
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. - PubMed
-
- Al-Khaldi A, Al-Sabti H, Galipeau J, et al. Therapeutic angiogenesis using autologous bone marrow stromal cells: Improved blood flow in a chronic limb ischemia model. Ann Thorac Surg. 2003;75:204–209. - PubMed
-
- Iwase T, Nagaya N, Fujii T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. - PubMed
-
- Moon MH, Kim SY, Kim YJ, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

