mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids
- PMID: 18511708
- PMCID: PMC2574528
- DOI: 10.1165/rcmb.2007-0460OC
mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids
Abstract
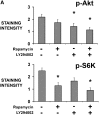
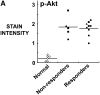
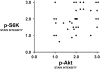
Solid tumors such as mesothelioma exhibit a stubborn resistance to apoptosis that may derive from survival pathways, such as PI3K/Akt/mTOR, that are activated in many tumors, including mesothelioma. To address the role of PI3K/Akt/mTOR, we used a novel approach to study mesothelioma ex vivo as tumor fragment spheroids. Freshly resected mesothelioma tissue from 15 different patients was grown in vitro as 1- to 2-mm-diameter fragments, exposed to apoptotic agents for 48 hours with or without PI3K/Akt/mTOR inhibitors, and doubly stained for cytokeratin and cleaved caspase 3 to identify apoptotic mesothelioma cells. Mesothelioma cells within the tumor spheroids exhibited striking resistance to apoptotic agents such as TRAIL plus gemcitabine that were highly effective against monolayers. In a majority of tumors (67%; 10 of 15), apoptotic resistance could be reduced by more than 50% by rapamycin, an mTOR inhibitor, but not by LY294002, a PI3K inhibitor. Responsiveness to rapamycin correlated with staining for the mTOR target, p-S6K, in the original tumor, but not for p-Akt. As confirmation of the role of mTOR, siRNA knockdown of S6K reproduced the effect of rapamycin in three rapamycin-responsive tumors. Finally, in 37 mesotheliomas on tissue microarray, p-S6K correlated only weakly with p-Akt, suggesting the existence of Akt-independent regulation of mTOR. We propose that mTOR mediates survival signals in many mesothelioma tumors. Inhibition of mTOR may provide a nontoxic adjunct to therapy directed against malignant mesothelioma, especially in those with high baseline expression of p-S6K.
Figures










References
-
- Wendel HG, Lowe SW. Reversing drug resistance in vivo. Cell Cycle 2004;3:847–849. - PubMed
-
- Smalley KSM, Lioni M, Herlyn M. Life isn't flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol 2006;42:242–247. - PubMed
-
- Pampaloni F, Reynoud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 2007;8:839–845. - PubMed
-
- Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen 2004;9:273–285. - PubMed
-
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

