A synthetic prostone activates apical chloride channels in A6 epithelial cells
- PMID: 18511742
- PMCID: PMC2519861
- DOI: 10.1152/ajpgi.00366.2007
A synthetic prostone activates apical chloride channels in A6 epithelial cells
Abstract
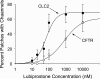
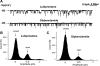
The bicyclic fatty acid lubiprostone (formerly known as SPI-0211) activates two types of anion channels in A6 cells. Both channel types are rarely, if ever, observed in untreated cells. The first channel type was activated at low concentrations of lubiprostone (<100 nM) in >80% of cell-attached patches and had a unit conductance of approximately 3-4 pS. The second channel type required higher concentrations (>100 nM) of lubiprostone to activate, was observed in approximately 30% of patches, and had a unit conductance of 8-9 pS. The properties of the first type of channel were consistent with ClC-2 and the second with CFTR. ClC-2's unit current strongly inwardly rectified that could be best fit by models of the channel with multiple energy barrier and multiple anion binding sites in the conductance pore. The open probability and mean open time of ClC-2 was voltage dependent, decreasing dramatically as the patches were depolarized. The order of anion selectivity for ClC-2 was Cl > Br > NO(3) > I > SCN, where SCN is thiocyanate. ClC-2 was a "double-barreled" channel favoring even numbers of levels over odd numbers as if the channel protein had two conductance pathways that opened independently of one another. The channel could be, at least, partially blocked by glibenclamide. The properties of the channel in A6 cells were indistinguishable from ClC-2 channels stably transfected in HEK293 cells. CFTR in the patches had a selectivity of Cl > Br >> NO(3) congruent with SCN congruent with I. It outwardly rectified as expected for a single-site anion channel. Because of its properties, ClC-2 is uniquely suitable to promote anion secretion with little anion reabsorption. CFTR, on the other hand, could promote either reabsorption or secretion depending on the anion driving forces.
Figures






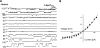



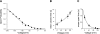





References
-
- Anonymous. Lubiprostone: RU 0211, SPI 0211. Drugs R D 6: 245–248, 2005. - PubMed
-
- Bali M, Lipecka J, Edelman A, Fritsch J. Regulation of ClC-2 chloride channels in T84 cells by TGF-alpha. Am J Physiol Cell Physiol 280: C1588–C1598, 2001. - PubMed
-
- Bear CE, Li C, Galley K, Wang Y, Garami E, Ramjeesingh M. Coupling of ATP hydrolysis with channel gating by purified, reconstituted CFTR. J Bioenerg Biomembr 29: 465–473, 1997. - PubMed
-
- Becchetti A, Kemendy AE, Stockand JD, Sariban-Sohraby S, Eaton DC. Methylation increases the open probability of the epithelial sodium channel in A6 epithelia. J Biol Chem 275: 16550–16559, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

