Oxidative innate immune defenses by Nox/Duox family NADPH oxidases
- PMID: 18511861
- PMCID: PMC2776633
- DOI: 10.1159/000136357
Oxidative innate immune defenses by Nox/Duox family NADPH oxidases
Abstract
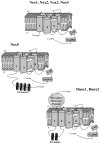
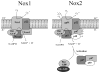
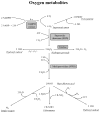
The importance of reactive oxygen species (ROS) in innate immunity was first recognized in professional phagocytes undergoing a 'respiratory burst'upon activation. This robust oxygen consumption is related to a superoxide-generating enzyme, the phagocytic NADPH oxidase (Nox2-based or phox). The oxidase is essential for microbial killing, since patients lacking a functional oxidase suffer from enhanced susceptibility to microbial infections. ROS derived from superoxide attack bacteria in the isolated niche of the neutrophil phagosome. The oxidase is electrogenic, alters ion currents across membranes, induces apoptosis, regulates cytokine production, influences gene expression, and promotes formation of extracellular traps. Recently, new homologues of Nox2 were discovered establishing the Nox family of NADPH oxidases that encompasses seven members. Nox1 is highly expressed in the colon epithelium, and can be induced by LPS or IFN- gamma. Nox4 was implicated in innate immunity since LPS induces Nox4-dependent ROS generation. Duox1 and Duox2 localize to the apical plasma membrane of epithelial cells in major airways, salivary glands, and the gastrointestinal tract, and provide extracellular hydrogen peroxide to lactoperoxidase to produce antimicrobial hypothiocyanite ions. Th1 and Th2 cytokines regulate expression of dual oxidases in human airways and may thereby act in host defense or in proinflammatory responses.
Figures


References
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. - PubMed
-
- Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279:51715–51718. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Leto TL, Geiszt M. Role of NADPH oxidases on host defense. Antioxid Redox Signal. 2006;8:1549–1561. - PubMed
-
- Segal BH, Leto TL, Malech HL, Gallin JI, Holland SM. Genetic, biochemical and clinical features of chronic granulomatous disease. Reviews in Molecular Medicine. 2000;79:170–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

