Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets
- PMID: 18511927
- PMCID: PMC2821027
- DOI: 10.1038/nrd2589
Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets
Abstract
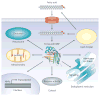
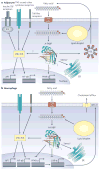
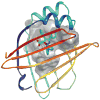
Lipids are vital components of many biological processes and crucial in the pathogenesis of numerous common diseases, but the specific mechanisms coupling intracellular lipids to biological targets and signalling pathways are not well understood. This is particularly the case for cells burdened with high lipid storage, trafficking and signalling capacity such as adipocytes and macrophages. Here, we discuss the central role of lipid chaperones--the fatty acid-binding proteins (FABPs)--in lipid-mediated biological processes and systemic metabolic homeostasis through the regulation of diverse lipid signals, and highlight their therapeutic significance. Pharmacological agents that modify FABP function may provide tissue-specific or cell-type-specific control of lipid signalling pathways, inflammatory responses and metabolic regulation, potentially providing a new class of drugs for diseases such as obesity, diabetes and atherosclerosis.
Figures
References
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. - PubMed
-
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. - PubMed
-
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1671–1875. - PubMed
-
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. - PubMed
-
- Haunerland NH, Spener F. Fatty acid-binding proteins — insights from genetic manipulations. Prog Lipid Res. 2004;43:328–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

