Functional and structural characterization of DR_0079 from Deinococcus radiodurans, a novel Nudix hydrolase with a preference for cytosine (deoxy)ribonucleoside 5'-Di- and triphosphates
- PMID: 18512963
- PMCID: PMC2867059
- DOI: 10.1021/bi800099d
Functional and structural characterization of DR_0079 from Deinococcus radiodurans, a novel Nudix hydrolase with a preference for cytosine (deoxy)ribonucleoside 5'-Di- and triphosphates
Abstract
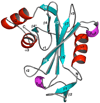
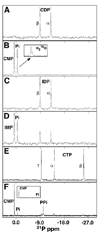
The genome of the extremely radiation resistant bacterium Deinococcus radiodurans encodes 21 Nudix hydrolases, of which only two have been characterized in detail. Here we report the activity and crystal structure for DR_0079, the first Nudix hydrolase observed to have a marked preference for cytosine ribonucleoside 5'-diphosphate (CDP) and cytosine ribonucleoside 5'-triphosphate (CTP). After CDP and CTP, the next most preferred substrates for DR_0079, with a relative activity of <50%, were the corresponding deoxyribose nucleotides, dCDP and dCTP. Hydrolase activity at the site of the phosphodiester bond was corroborated using (31)P NMR spectroscopy to follow the phosphorus resonances for three substrates, CDP, IDP, and CTP, and their hydrolysis products, CMP + P(i), IMP + P(i), and CMP + PP(i), respectively. Nucleophilic substitution at the beta-phosphorus of CDP and CTP was established, using (31)P NMR spectroscopy, by the appearance of an upfield-shifted P(i) resonance and line-broadened PP(i) resonance, respectively, when the hydrolysis was performed in 40% H(2)(18)O-enriched water. The optimal activity for CDP was at pH 9.0-9.5 with the reaction requiring divalent metal cation (Mg(2+) > Mn(2+) > Co(2+)). The biochemical data are discussed with reference to the crystal structure for DR_0079 that was determined in the metal-free form at 1.9 A resolution. The protein contains nine beta-strands, three alpha-helices, and two 3(10)-helices organized into three subdomains: an N-terminal beta-sheet, a central Nudix core, and a C-terminal helix-turn-helix motif. As observed for all known structures of Nudix hydrolases, the alpha-helix of the "Nudix box" is one of two helices that sandwich a "four-strand" mixed beta-sheet. To identify residues potentially involved in metal and substrate binding, NMR chemical shift mapping experiments were performed on (15)N-labeled DR_0079 with the paramagnetic divalent cation Co(2+) and the nonhydrolyzable substrate thymidine 5'-O-(alpha,beta-methylenediphosphate) and the results mapped onto the crystal structure.
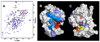
Figures

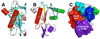
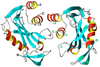


References
-
- Abeygunawardana C, Weber DJ, Gittis AG, Frick DN, Lin J, Miller AF, Bessman MJ, Mildvan AS. Solution structure of the MutT enzyme, a nucleoside triphosphate pyrophosphohydrolase. Biochemistry. 1995;34:14997–15005. - PubMed
-
- Bessman MJ, Frick N, O’Handley SF. The MutT proteins or "Nudix" hydrolases, a family of versatile, widely distributed, "housecleaning" enzymes. J. Biol. Chem. 1996;271:25059–25062. - PubMed
-
- Dunn CA, O’Handley SF, Frick DN, Bessman MJ. Studies on the ADP-ribose pyrophosphatase subfamily of the nudix hydrolases and tentative identification of trgB, a gene associated with tellurite resistance. J. Biol. Chem. 1999;274:32318–32324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

