Neuronal mechanisms of visual stability
- PMID: 18513781
- PMCID: PMC2556215
- DOI: 10.1016/j.visres.2008.03.021
Neuronal mechanisms of visual stability
Abstract
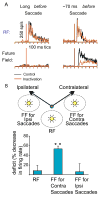
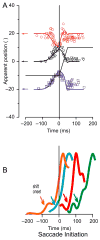
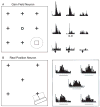
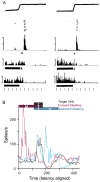
Human vision is stable and continuous in spite of the incessant interruptions produced by saccadic eye movements. These rapid eye movements serve vision by directing the high resolution fovea rapidly from one part of the visual scene to another. They should detract from vision because they generate two major problems: displacement of the retinal image with each saccade and blurring of the image during the saccade. This review considers the substantial advances in understanding the neuronal mechanisms underlying this visual stability derived primarily from neuronal recording and inactivation studies in the monkey, an excellent model for systems in the human brain. For the first problem, saccadic displacement, two neuronal candidates are salient. First are the neurons in frontal and parietal cortex with shifting receptive fields that provide anticipatory activity with each saccade and are driven by a corollary discharge. These could provide the mechanism for a retinotopic hypothesis of visual stability and possibly for a transsaccadic memory hypothesis, The second neuronal mechanism is provided by neurons whose visual response is modulated by eye position (gain field neurons) or are largely independent of eye position (real position neurons), and these neurons could provide the basis for a spatiotopic hypothesis. For the second problem, saccadic suppression, visual masking and corollary discharge are well established mechanisms, and possible neuronal correlates have been identified for each.
Figures







References
-
- Andersen RA. Visual and eye movement functions of the posterior parietal cortex. Annu Rev Neurosci. 1989;12:377–403. - PubMed
-
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230(4724):456–458. - PubMed
-
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. - PubMed
-
- Baldauf D, Wolf M, Deubel H. Deployment of visual attention before sequences of goal-directed hand movements. Vision Res. 2006;46(26):4355–4374. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

