The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens
- PMID: 18513998
- PMCID: PMC2601674
- DOI: 10.1016/j.immuni.2008.03.019
The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens
Abstract
Synergistic engagement of the B cell receptor (BCR) and Toll-like receptor 9 (TLR9) in response to DNA-containing antigens underlies the production of many autoantibodies in systemic autoimmune diseases. However, the molecular basis of this synergistic engagement is not known. Given that these receptors are spatially segregated, with the BCR on the cell surface and TLR9 in endocytic vesicles, achieving synergy must involve unique mechanisms. We show that upon antigen binding, the BCR initiates signaling at the plasma membrane and continues to signal to activate MAP kinases as it traffics to autophagosome-like compartments. The internalized BCR signals through a phospholipase-D-dependent pathway to recruit TLR9-containing endosomes to the autophagosome via the microtubular network. The recruitment of TLR9 to the autophagosomes was necessary for hyperactivation of MAP kinases. This unique mechanism for BCR-induced TLR9 recruitment resulting in B cells hyperresponses may provide new targets for therapeutics for autoimmune diseases.
Figures



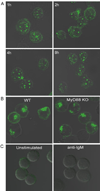
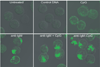
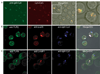

Comment in
-
Bridging Toll-like- and B cell-receptor signaling: meet me at the autophagosome.Immunity. 2008 Jun;28(6):729-31. doi: 10.1016/j.immuni.2008.05.006. Immunity. 2008. PMID: 18549794
References
-
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. - PubMed
-
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. - PubMed
-
- Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. - PubMed
-
- Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. - PubMed
-
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

