Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy
- PMID: 18514161
- PMCID: PMC2427288
- DOI: 10.1016/j.ajhg.2008.04.020
Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy
Abstract
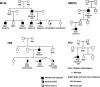
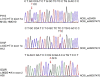
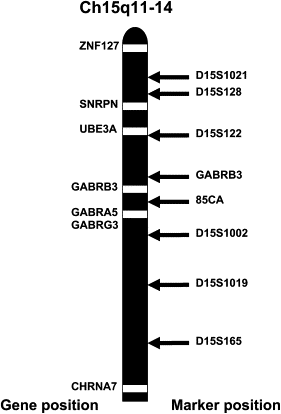
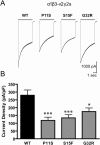
Childhood absence epilepsy (CAE) accounts for 10% to 12% of epilepsy in children under 16 years of age. We screened for mutations in the GABA(A) receptor (GABAR) beta 3 subunit gene (GABRB3) in 48 probands and families with remitting CAE. We found that four out of 48 families (8%) had mutations in GABRB3. One heterozygous missense mutation (P11S) in exon 1a segregated with four CAE-affected persons in one multiplex, two-generation Mexican family. P11S was also found in a singleton from Mexico. Another heterozygous missense mutation (S15F) was present in a singleton from Honduras. An exon 2 heterozygous missense mutation (G32R) was present in two CAE-affected persons and two persons affected with EEG-recorded spike and/or sharp wave in a two-generation Honduran family. All mutations were absent in 630 controls. We studied functions and possible pathogenicity by expressing mutations in HeLa cells with the use of Western blots and an in vitro translation and translocation system. Expression levels did not differ from those of controls, but all mutations showed hyperglycosylation in the in vitro translation and translocation system with canine microsomes. Functional analysis of human GABA(A) receptors (alpha 1 beta 3-v2 gamma 2S, alpha 1 beta 3-v2[P11S]gamma 2S, alpha 1 beta 3-v2[S15F]gamma 2S, and alpha 1 beta 3-v2[G32R]gamma 2S) transiently expressed in HEK293T cells with the use of rapid agonist application showed that each amino acid transversion in the beta 3-v2 subunit (P11S, S15F, and G32R) reduced GABA-evoked current density from whole cells. Mutated beta 3 subunit protein could thus cause absence seizures through a gain in glycosylation of mutated exon 1a and exon 2, affecting maturation and trafficking of GABAR from endoplasmic reticulum to cell surface and resulting in reduced GABA-evoked currents.
Figures



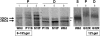

References
-
- Fong G.C.Y., Shah P.U., Gee M.N., Serratosa J.M., Castroviejo I.P., Khan S., Ravat S.H., Mani J., Medina M.T., Delgado-Escueta A.V. Childhood absence epilepsy with tonic-clonic seizures and 3–4-Hz spike and multispike-slow wave complexes: linkage to chromosome 8q24. Am. J. Hum. Genet. 1998;63:1117–1129. - PMC - PubMed
-
- Wallace R.H., Marini C., Petrou S., Harkin L.A., Owser D.N., Panchal R.G., Williams D.A., Sutherland G.R., Mulley J.C., Berkovic S.F. Mutant GABA(A)receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28:49–52. - PubMed
-
- Haug K., Warnstedt M., Alekov A.K., Sander T., Ramírez A., Poser B., Maljevic S., Hebeisen S., Kubisch C., Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat. Genet. 2003;33:527–532. - PubMed
-
- Maljevic S., Krampfl K., Cobilanschi J., Tilgen N., Beyer S., Weber Y.G., Schlesinger F., Ursu D., Melzer W., Cossette P. A mutation in the GABA(A) receptor alpha(1)-subunit is associated with absence epilepsy. Ann. Neurol. 2006;59:983–987. - PubMed
-
- Callenbach P.M., Geerts A.T., Arts W.F., van Donselaar C.A., Peters A.C., Stroink H., Brouwer O.F. Familial occurrence of epilepsy in children with newly diagnosed multiple seizures: Dutch Study of Epilepsy in Childhood. Epilepsia. 1998;39:331–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

