Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice
- PMID: 18514180
- PMCID: PMC2536689
- DOI: 10.1016/j.ydbio.2008.04.024
Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice
Abstract
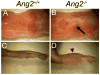
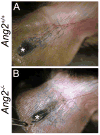
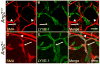
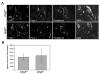
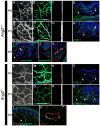
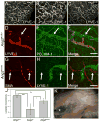
Molecular mechanisms regulating the remodeling of the lymphatic vasculature from an immature plexus of vessels to a hierarchal network of initial and collecting lymphatics are not well understood. One gene thought to be important for this process is Angiopoietin-2 (Ang-2). Ang2(-/-) mice have previously been reported to exhibit an abnormal lymphatic phenotype but the precise nature of the lymphatic defects and the underlying mechanisms have yet to be defined. Here we demonstrate by whole-mount immunofluorescence staining of ear skin and mesentery that lymphatic vessels in Ang2(-/-) mice fail to mature and do not exhibit a collecting vessel phenotype. Furthermore, dermal lymphatic vessels in Ang2(-/-) pups prematurely recruit smooth muscle cells and do not undergo proper postnatal remodeling. In contrast, Ang2 knock-out Ang1 knock-in mice do develop a hierarchal lymphatic vasculature, suggesting that activation of Tie-2 is required for normal lymphatic development. Taken together, this work pinpoints a specific lymphatic defect of Ang2(-/-) mice and further defines the sequential steps in lymphatic vessel remodeling.
Figures





References
-
- Dellinger MT, Hunter RJ, Bernas MJ, Witte MH, Erickson RP. Chy-3 mice are Vegfc haploinsufficient and exhibit defective dermal superficial to deep lymphatic transition and dermal lymphatic hypoplasia. Dev Dyn. 2007;236:2346–2355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

