Structural basis for the regulation of muscle contraction by troponin and tropomyosin
- PMID: 18514658
- PMCID: PMC2483953
- DOI: 10.1016/j.jmb.2008.04.062
Structural basis for the regulation of muscle contraction by troponin and tropomyosin
Abstract
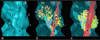
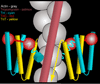
The molecular switching mechanism governing skeletal and cardiac muscle contraction couples the binding of Ca2+ on troponin to the movement of tropomyosin on actin filaments. Despite years of investigation, this mechanism remains unclear because it has not yet been possible to directly assess the structural influence of troponin on tropomyosin that causes actin filaments, and hence myosin-crossbridge cycling and contraction, to switch on and off. A C-terminal domain of troponin I is thought to be intimately involved in inducing tropomyosin movement to an inhibitory position that blocks myosin-crossbridge interaction. Release of this regulatory, latching domain from actin after Ca2+ binding to TnC (the Ca2+ sensor of troponin that relieves inhibition) presumably allows tropomyosin movement away from the inhibitory position on actin, thus initiating contraction. However, the structural interactions of the regulatory domain of TnI (the "inhibitory" subunit of troponin) with tropomyosin and actin that cause tropomyosin movement are unknown, and thus, the regulatory process is not well defined. Here, thin filaments were labeled with an engineered construct representing C-terminal TnI, and then, 3D electron microscopy was used to resolve where troponin is anchored on actin-tropomyosin. Electron microscopy reconstruction showed how TnI binding to both actin and tropomyosin at low Ca2+ competes with tropomyosin for a common site on actin and drives tropomyosin movement to a constrained, relaxing position to inhibit myosin-crossbridge association. Thus, the observations reported reveal the structural mechanism responsible for troponin-tropomyosin-mediated steric interference of actin-myosin interaction that regulates muscle contraction.
Figures



References
-
- Huxley HE. Structural changes in actin- and myosin-containing filaments during contraction. Cold Spring Harbor Symp. Quant. Biol. 1972;37:361–376.
-
- Haselgrove JC. X-ray evidence for a conformational change in actin-containing filaments of vertebrate striated muscle. Cold Spring Harbor Symp. Quant. Biol. 1972;37:341–352.
-
- Parry DAD, Squire JM. Structural role of tropomyosin in muscle regulation: Analysis of the X-ray patterns from relaxed and contracting muscles. J. Mol. Biol. 1973;75:33–55. - PubMed
-
- Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three dimensional reconstruction. Nature. 1994;368:65–67. - PubMed
-
- Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997;266:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

