The rotary mechanism of the ATP synthase
- PMID: 18515057
- PMCID: PMC2581510
- DOI: 10.1016/j.abb.2008.05.004
The rotary mechanism of the ATP synthase
Abstract
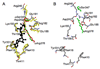
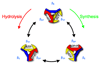
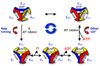
The F0F1 ATP synthase is a large complex of at least 22 subunits, more than half of which are in the membranous F0 sector. This nearly ubiquitous transporter is responsible for the majority of ATP synthesis in oxidative and photo-phosphorylation, and its overall structure and mechanism have remained conserved throughout evolution. Most examples utilize the proton motive force to drive ATP synthesis except for a few bacteria, which use a sodium motive force. A remarkable feature of the complex is the rotary movement of an assembly of subunits that plays essential roles in both transport and catalytic mechanisms. This review addresses the role of rotation in catalysis of ATP synthesis/hydrolysis and the transport of protons or sodium.
Figures
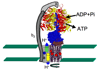

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

