Hedgehog signaling is required for effective regeneration of exocrine pancreas
- PMID: 18515092
- PMCID: PMC2666349
- DOI: 10.1053/j.gastro.2008.04.011
Hedgehog signaling is required for effective regeneration of exocrine pancreas
Abstract
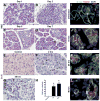
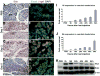
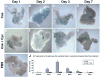
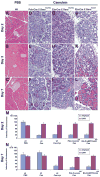
Although both endocrine and the exocrine pancreas display a significant capacity for tissue regeneration and renewal, the existence of progenitor cells in the adult pancreas remains uncertain. Using a model of cerulein-mediated injury and repair, we demonstrate that mature exocrine cells, defined by expression of an Elastase1 promoter, actively contribute to regenerating pancreatic epithelium through formation of metaplastic ductal intermediates. Acinar cell regeneration is associated with activation of Hedgehog (Hh) signaling, as assessed by up-regulated expression of multiple pathway components, as well as activation of a Ptch-lacZ reporter allele. Using both pharmacologic and genetic techniques, we also show that the ability of mature exocrine cells to accomplish pancreatic regeneration is impaired by blockade of Hh signaling. Specifically, attenuated regeneration in the absence of an intact Hh pathway is characterized by persistence of metaplastic epithelium expressing markers of pancreatic progenitor cells, suggesting an inhibition of redifferentiation into mature exocrine cells. Given the known role of Hh signaling in exocrine pancreatic cancer, these findings may provide a mechanistic link between injury-induced activation of pancreatic progenitors and subsequent pancreatic neoplasia.
Conflict of interest statement
Conflicts of interest: The authors declare no competing financial conflicts of interest.
Figures

Comment in
-
Hedgehog spikes pancreas regeneration.Gastroenterology. 2008 Aug;135(2):347-51. doi: 10.1053/j.gastro.2008.06.063. Epub 2008 Jul 11. Gastroenterology. 2008. PMID: 18619970 No abstract available.
Similar articles
-
Hedgehog spikes pancreas regeneration.Gastroenterology. 2008 Aug;135(2):347-51. doi: 10.1053/j.gastro.2008.06.063. Epub 2008 Jul 11. Gastroenterology. 2008. PMID: 18619970 No abstract available.
-
NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas.Gastroenterology. 2015 May;148(5):1024-1034.e9. doi: 10.1053/j.gastro.2015.01.033. Epub 2015 Jan 23. Gastroenterology. 2015. PMID: 25623042 Free PMC article.
-
Bmi1 is required for regeneration of the exocrine pancreas in mice.Gastroenterology. 2012 Sep;143(3):821-831.e2. doi: 10.1053/j.gastro.2012.05.009. Epub 2012 May 17. Gastroenterology. 2012. PMID: 22609312 Free PMC article.
-
Regeneration and repair of the exocrine pancreas.Annu Rev Physiol. 2015;77:229-49. doi: 10.1146/annurev-physiol-021014-071727. Epub 2014 Oct 24. Annu Rev Physiol. 2015. PMID: 25386992 Free PMC article. Review.
-
Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells.Semin Cell Dev Biol. 2012 Aug;23(6):711-9. doi: 10.1016/j.semcdb.2012.06.008. Epub 2012 Jun 26. Semin Cell Dev Biol. 2012. PMID: 22743232 Free PMC article. Review.
Cited by
-
Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma.Cancer Cell. 2012 Dec 11;22(6):737-50. doi: 10.1016/j.ccr.2012.10.025. Epub 2012 Nov 29. Cancer Cell. 2012. PMID: 23201164 Free PMC article.
-
Novel Approaches in Non-Melanoma Skin Cancers-A Focus on Hedgehog Pathway in Basal Cell Carcinoma (BCC).Cells. 2022 Oct 13;11(20):3210. doi: 10.3390/cells11203210. Cells. 2022. PMID: 36291078 Free PMC article. Review.
-
Retinoid signaling in pancreatic cancer, injury and regeneration.PLoS One. 2011;6(12):e29075. doi: 10.1371/journal.pone.0029075. Epub 2011 Dec 29. PLoS One. 2011. PMID: 22220202 Free PMC article.
-
Primary cilia regulate Gli/Hedgehog activation in pancreas.Proc Natl Acad Sci U S A. 2010 Jun 1;107(22):10109-14. doi: 10.1073/pnas.0909900107. Epub 2010 May 17. Proc Natl Acad Sci U S A. 2010. PMID: 20479231 Free PMC article.
-
Myeloid Cell-Derived HB-EGF Drives Tissue Recovery After Pancreatitis.Cell Mol Gastroenterol Hepatol. 2019;8(2):173-192. doi: 10.1016/j.jcmgh.2019.05.006. Epub 2019 May 21. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31125624 Free PMC article.
References
-
- Alison MR, Poulsom R, Forbes SJ. Update on hepatic stem cells. Liver. 2001;21:367–373. - PubMed
-
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
-
- Dor Y, Brown J, Martinez OI, et al. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
-
- Jensen JN, Cameron E, Garay MV, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

