Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells
- PMID: 18515280
- PMCID: PMC2500214
- DOI: 10.1093/carcin/bgn124
Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells
Abstract
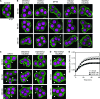
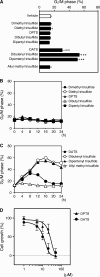
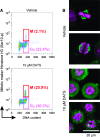
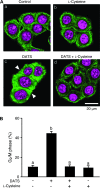
Alk(en)yl trisulfides (R-SSS-R') are organosulfur compounds produced by crushed garlic and other Allium vegetables. We found that these compounds exhibit potent anticancer effects through the reaction with microtubules, causing cell cycle arrest. Nine alk(en)yl trisulfides including dimethyl trisulfide, diethyl trisulfide, dipropyl trisulfide (DPTS), dibutyl trisulfide, dipentyl trisulfide, diallyl trisulfide (DATS), dibutenyl trisulfide, dipentenyl trisulfide and allyl methyl trisulfide were synthesized and added to cultures of HT-29 human colon cancer cells at a concentration of 10 muM. The trisulfides with alkenyl groups such as DATS, but not those with alkyl groups, induced rapid microtubule disassembly at 30-60 min as well as cell cycle arrest during the mitotic phase approximately at 4 h after the treatment. Both DATS-induced microtubule disassembly and the cell cycle arrest were cancelled by the simultaneous treatment of the cancer cells with 2 mM L-cysteine, glutathione (GSH) or N-acetyl-L-cysteine. Reciprocally, L-buthionine-(S,R)-sulfoximine (500 muM), an inhibitor of GSH synthesis, enhanced the power of DATS in inducing the cell cycle arrest. These results indicate that alk(en)yl trisulfide react with sulfhydryl groups in cysteine residues of cellular proteins such as microtubule proteins. Thus, the present study provides evidence that trisulfides with alkenyl groups have potent anticancer activities, at least in part, directed toward microtubules. These findings suggest that alkenyl trisulfides and their structurally related compounds may provide novel and effective anticancer agents.
Figures


References
-
- Ross SA, et al. Allyl sulfur compounds from garlic modulate aberrant crypt formation. J. Nutr. 2006;136:852S–854S. - PubMed
-
- Sakamoto K, et al. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr. Cancer. 1997;29:152–156. - PubMed
-
- Filomeni G, et al. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63:5940–5949. - PubMed
-
- Xiao D, et al. Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH2-terminal kinase 1 activation. Cancer Res. 2003;63:6825–6837. - PubMed
-
- Xiao D, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

