The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts
- PMID: 18515355
- PMCID: PMC2516982
- DOI: 10.1074/jbc.R800023200
The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts
Figures



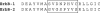
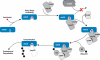
References
-
- Pratt, W. B., and Toft, D. O. (1997) Endocr. Rev. 18 306–360 - PubMed
-
- Pratt, W. B., and Toft, D. O. (2003) Exp. Biol. Med. 222 111–133 - PubMed
-
- Glickman, M. H., and Ciechanover, A. (2002) Physiol. Rev. 82 373–428 - PubMed
-
- Cyr, D. M., Hohfeld, J., and Patterson, C. (2002) Trends Biochem. Sci. 27 368–375 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

