Not only enthalpy: large entropy contribution to ion permeation barriers in single-file channels
- PMID: 18515367
- PMCID: PMC2517043
- DOI: 10.1529/biophysj.108.130609
Not only enthalpy: large entropy contribution to ion permeation barriers in single-file channels
Abstract
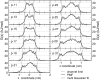
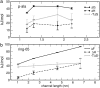
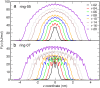
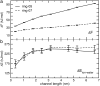
The effect of channel length on the barrier for potassium ion permeation through single-file channels has been studied by means of all-atom molecular dynamics simulations. Using series of peptidic gramicidin-like and simplified ring-structured channels, both embedded in model membranes, we obtained two distinct types of behavior: saturation of the central free energy barriers for peptidic channels and a linear increase in simplified ring-structured channels with increasing channel length. The saturation of the central free energy barrier for the peptidic channels occurs at relatively short lengths, and it is correlated with the desolvation from the bulk water. Remarkably, decomposition of free energy barriers into enthalpic and entropic terms reveals an entropic cost for ion permeation. Furthermore, this entropic cost dominates the ion permeation free energy barrier, since the corresponding free energy contribution is higher than the enthalpic barrier. We conclude that the length dependence of the free energy is enthalpy-dominated, but the entropy is the major contribution to the permeation barrier. The decrease in rotational water motion and the reduction of channel mobility are putative origins for the overall entropic penalty.
Figures



References
-
- Wallace, B. A. 2000. Common structural features in gramicidin and other ion channels. Bioessays. 22:227–234. - PubMed
-
- Hille, B. 2001. Ion Channels of Excitable Membranes, 3rd Ed. Sinauer, Sunderland, MA.
-
- Gouaux, E., and R. Mackinnon. 2005. Principles of selective ion transport in channels and pumps. Science. 310:1461–1465. - PubMed
-
- Doyle, D. A., J. M. Cabral, R. A. Pfuetzner, A. L. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. - PubMed
-
- Zhou, Y., J. H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

