Prediction of protein solubility from calculation of transfer free energy
- PMID: 18515380
- PMCID: PMC2527251
- DOI: 10.1529/biophysj.107.127746
Prediction of protein solubility from calculation of transfer free energy
Abstract
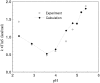
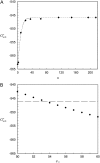
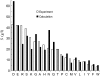
Solubility plays a major role in protein purification, and has serious implications in many diseases. We studied the effects of pH and mutations on protein solubility by calculating the transfer free energy from the condensed phase to the solution phase. The condensed phase was modeled as an implicit solvent, with a dielectric constant lower than that of water. To account for the effects of pH, the protonation states of titratable side chains were sampled by running constant-pH molecular dynamics simulations. Conformations were then selected for calculations of the electrostatic solvation energy: once for the condensed phase, and once for the solution phase. The average transfer free energy from the condensed phase to the solution phase was found to predict reasonably well the variations in solubility of ribonuclease Sa and insulin with pH. This treatment of electrostatic contributions combined with a similar approach for nonelectrostatic contributions led to a quantitative rationalization of the effects of point mutations on the solubility of ribonuclease Sa. This study provides valuable insights into the physical basis of protein solubility.
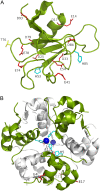
Figures



References
-
- Howe, P. W. A. 2004. A straight-forward method of optimising protein solubility for NMR. J. Biomol. NMR. 30:283–286. - PubMed
-
- Jancarik, J., R. Pufan, C. Hong, S.-H. Kim, and R. Kim. 2004. Optimum solubility (OS) screening: an efficient method to optimize buffer conditions for homogeneity and crystallization of proteins. Acta Crystallogr. D Biol. Crystallogr. 60:1670–1673. - PubMed
-
- Machida, S., Y. Yu, S. P. Singh, J.-D. Kim, K. Hayashi, and Y. Kawata. 1998. Overproduction of β-glucosidase in active form by an Escherichia coli system coexpressing the chaperonin GroEL/ES. FEMS Microbiol. Lett. 159:41–46. - PubMed
-
- Zhou, P., A. A. Lugovskoy, and G. Wagner. 2001. A solubility-enhancement tag (SET) for NMR studies of poorly behaving proteins. J. Biomol. NMR. 20:11–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

