On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface
- PMID: 18515388
- PMCID: PMC2517020
- DOI: 10.1529/biophysj.107.127423
On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface
Abstract
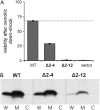
The mechanosensitive channel of large conductance, MscL, serves as a biological emergency release valve protecting bacteria from acute osmotic downshock and is to date the best characterized mechanosensitive channel. A well-recognized and supported model for Escherichia coli MscL gating proposes that the N-terminal 11 amino acids of this protein form a bundle of amphipathic helices in the closed state that functionally serves as a cytoplasmic second gate. However, a recently reexamined crystal structure of a closed state of the Mycobacterium tuberculosis MscL shows these helices running along the cytoplasmic surface of the membrane. Thus, it is unclear if one structural model is correct or if they both reflect valid closed states. Here, we have systematically reevaluated this region utilizing cysteine-scanning, in vivo functional characterization, in vivo SCAM, electrophysiological studies, and disulfide-trapping experiments. The disulfide-trapping pattern and functional studies do not support the helical bundle and second-gate hypothesis but correlate well with the proposed structure for M. tuberculosis MscL. We propose a functional model that is consistent with the collective data.
Figures




References
-
- Hamill, O. P., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. - PubMed
-
- Niu, W., and F. Sachs. 2003. Dynamic properties of strech-activated K+ channels in adult rat atrial myocytes. Prog. Biophys. Mol. Biol. 82:121–135. - PubMed
-
- Sukharev, S., and D. P. Corey. 2004. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE. 2004:re4. - PubMed
-
- Bass, R. B., P. Strop, M. Barclay, and D. C. Rees. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 298:1582–1587. - PubMed
-
- Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science. 282:2220–2226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

