Four-scale description of membrane sculpting by BAR domains
- PMID: 18515394
- PMCID: PMC2527247
- DOI: 10.1529/biophysj.108.132563
Four-scale description of membrane sculpting by BAR domains
Abstract
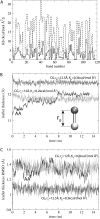
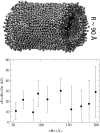
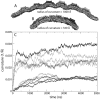
BAR domains are proteins that sense and sculpt curved membranes in cells, furnishing a relatively well-studied example of mechanisms employed in cellular morphogenesis. We report a computational study of membrane bending by BAR domains at four levels of resolution, described by 1), all-atom molecular dynamics; 2), residue-based coarse-graining (resolving single amino acids and lipid molecules); 3), shape-based coarse-graining (resolving overall protein and membrane shapes); and 4), a continuum elastic membrane model. Membrane sculpting performed by BAR domains collectively is observed in agreement with experiments. Different arrangements of BAR domains on the membrane surface are found to lead to distinct membrane curvatures and bending dynamics.
Figures










References
-
- Zimmerberg, J., and M. M. Kozlov. 2006. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7:9–19. - PubMed
-
- Rothman, J. E., and L. Orci. 1996. Budding vesicles in living cells. Sci. Am. 274:70–75. - PubMed
-
- Lecuit, T., and F. Pilot. 2003. Developmental control of cell morphogenesis: a focus on membrane growth. Nat. Cell Biol. 5:103–108. - PubMed
-
- Marsh, M., and H. T. McMahon. 1999. The structural era of endocytosis. Science. 285:215–220. - PubMed
-
- Kirchhausen, T. 2000. Clathrin. Annu. Rev. Biochem. 69:699–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

