Structure and host-cell interaction of SH1, a membrane-containing, halophilic euryarchaeal virus
- PMID: 18515426
- PMCID: PMC2430350
- DOI: 10.1073/pnas.0801758105
Structure and host-cell interaction of SH1, a membrane-containing, halophilic euryarchaeal virus
Abstract
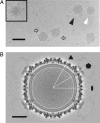
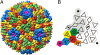
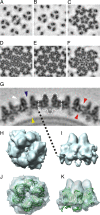
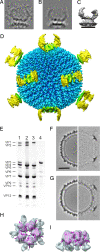
The Archaea, and the viruses that infect them, are the least well understood of all of the three domains of life. They often grow in extreme conditions such as hypersaline lakes and sulfuric hot springs. Only rare glimpses have been gained into the structures of archaeal viruses. Here, we report the subnanometer resolution structure of a recently isolated, hypersalinic, membrane-containing, euryarchaeal virus, SH1, in which different viral proteins can be localized. The results indicate that SH1 has a complex capsid formed from single beta-barrels, an important missing link in hypotheses on viral capsid protein evolution. Unusual, symmetry-mismatched spikes seem to play a role in host adsorption. They are connected to highly organized membrane proteins providing a platform for capsid assembly and potential machinery for host infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Benson SD, Bamford JK, Bamford DH, Burnett RM. Does common architecture reveal a viral lineage spanning all three domains of life? Mol Cell. 2004;16:673–685. - PubMed
-
- Benson SD, Bamford JK, Bamford DH, Burnett RM. Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell. 1999;98:825–833. - PubMed
-
- Laurinmäki PA, Huiskonen JT, Bamford DH, Butcher SJ. Membrane proteins modulate the bilayer curvature in the bacterial virus Bam35. Structure (London) 2005;13:1819–1828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

