A nonneural epithelial domain of embryonic cranial neural folds gives rise to ectomesenchyme
- PMID: 18515427
- PMCID: PMC2408482
- DOI: 10.1073/pnas.0711344105
A nonneural epithelial domain of embryonic cranial neural folds gives rise to ectomesenchyme
Abstract
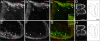
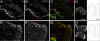
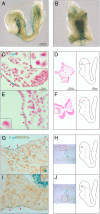
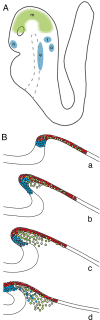
The neural crest is generally believed to be the embryonic source of skeletogenic mesenchyme (ectomesenchyme) in the vertebrate head and other derivatives, including pigment cells and neurons and glia of the peripheral nervous system. Although classical transplantation experiments leading to this conclusion assumed that embryonic neural folds were homogeneous epithelia, we reported that embryonic cranial neural folds contain spatially and phenotypically distinct domains, including a lateral nonneural domain with cells that coexpress E-cadherin and PDGFRalpha and a thickened mediodorsal neuroepithelial domain where these proteins are reduced or absent. We now show that Wnt1-Cre is expressed in the lateral nonneural epithelium of rostral neural folds and that cells coexpressing Cre-recombinase and PDGFRalpha delaminate precociously from some of this nonneural epithelium. We also show that ectomesenchymal cells exhibit beta-galactosidase activity in embryos heterozygous for an Ecad-lacZ reporter knock- in allele. We conclude that a lateral nonneural domain of the neural fold epithelium, which we call "metablast," is a source of ectomesenchyme distinct from the neural crest. We suggest that closer analysis of the origin of ectomesenchyme might help to understand (i) the molecular-genetic regulation of development of both neural crest and ectomesenchyme lineages; (ii) the early developmental origin of skeletogenic and connective tissue mesenchyme in the vertebrate head; and (iii) the presumed origin of head and branchial arch skeletal and connective tissue structures during vertebrate evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev Mol Cell Biol. 2006;7:131–142. - PubMed
-
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: Analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. - PubMed
-
- Hall BK, Hörstadius S. The Neural Crest. London: Oxford Univ Press; 1988.
-
- Le Douarin NM, Kalcheim C. The Neural Crest. 2nd Ed. Cambridge Univ Press; 2000.
-
- Weston JA, et al. Neural Crest and the origin of ectomesenchyme: Neural fold heterogeneity suggests an alternative hypothesis. Devel Dyn. 2004;229:118–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

