Temporal release of fatty acids and sugars in the spermosphere: impacts on Enterobacter cloacae-induced biological control
- PMID: 18515478
- PMCID: PMC2493187
- DOI: 10.1128/AEM.00264-08
Temporal release of fatty acids and sugars in the spermosphere: impacts on Enterobacter cloacae-induced biological control
Abstract
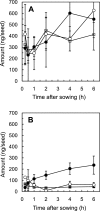
The aim of this study was to determine the temporal release of fatty acids and sugars from corn and cucumber seeds during the early stages of seed germination in order to establish whether sugars found in exudate can prevent exudate fatty acid degradation by Enterobacter cloacae. Both saturated (long-chain saturated fatty acids [LCSFA]) and unsaturated (long-chain unsaturated fatty acids [LCUFA]) fatty acids were detected in corn and cucumber seed exudates within 15 min after seed sowing. LCSFA and LCUFA were released at a rate of 26.1 and 6.44 ng/min/seed by corn and cucumber seeds, respectively. The unsaturated portion of the total fatty acid pool from both plant species contained primarily oleic and linoleic acids, and these fatty acids were released at a combined rate of 6.6 and 0.67 ng/min/seed from corn and cucumber, respectively. In the absence of seed exudate sugars, E. cloacae degraded linoleic acid at rates of 29 to 39 ng/min, exceeding the rate of total fatty acid release from seeds. Sugars constituted a significant percentage of corn seed exudate, accounting for 41% of the total dry seed weight. Only 5% of cucumber seed exudate was comprised of sugars. Glucose, fructose, and sucrose were the most abundant sugars present in seed exudate from both plant species. Corn seeds released a total of 137 microg/seed of these three sugars within 30 min of sowing, whereas cucumber seeds released 0.83 microg/seed within the same time frame. Levels of glucose, fructose, and sucrose found in corn seed exudate (90 to 342 microg) reduced the rate of linoleic acid degradation by E. cloacae to 7.5 to 8.8 ng/min in the presence of either sugar, leaving sufficient concentrations of linoleic acid to activate Pythium ultimum sporangia Our results demonstrate that elevated levels of sugars in the corn spermosphere can prevent the degradation of LCUFA by E. cloacae, leading to its failure to suppress P. ultimum sporangial activation, germination, and subsequent disease development.
Figures






Similar articles
-
Differential interference with Pythium ultimum sporangial activation and germination by Enterobacter cloacae in the corn and cucumber spermospheres.Appl Environ Microbiol. 2008 Jul;74(14):4285-91. doi: 10.1128/AEM.00263-08. Epub 2008 May 30. Appl Environ Microbiol. 2008. PMID: 18515482 Free PMC article.
-
Microbial-induced carbon competition in the spermosphere leads to pathogen and disease suppression in a municipal biosolids compost.Phytopathology. 2012 Jun;102(6):588-96. doi: 10.1094/PHYTO-08-11-0241. Phytopathology. 2012. PMID: 22352306
-
Differential inactivation of seed exudate stimulation of Pythium ultimum sporangium germination by Enterobacter cloacae influences biological control efficacy on different plant species.Appl Environ Microbiol. 2003 Feb;69(2):1114-20. doi: 10.1128/AEM.69.2.1114-1120.2003. Appl Environ Microbiol. 2003. PMID: 12571037 Free PMC article.
-
Sugars as signal molecules in plant seed development.Biol Chem. 1999 Jul-Aug;380(7-8):937-44. doi: 10.1515/BC.1999.116. Biol Chem. 1999. PMID: 10494845 Review.
-
The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease.Prog Cardiovasc Dis. 2016 Mar-Apr;58(5):464-72. doi: 10.1016/j.pcad.2015.11.006. Epub 2015 Nov 14. Prog Cardiovasc Dis. 2016. PMID: 26586275 Free PMC article. Review.
Cited by
-
Water-soluble exudates from seeds of Kochia scoparia exhibit antifungal activity against Colletotrichum graminicola.PLoS One. 2019 Jun 19;14(6):e0218104. doi: 10.1371/journal.pone.0218104. eCollection 2019. PLoS One. 2019. PMID: 31216294 Free PMC article.
-
Impact of thermal seed treatment on spermosphere microbiome, metabolome and viability of winter wheat.Sci Rep. 2024 Nov 8;14(1):27197. doi: 10.1038/s41598-024-78575-0. Sci Rep. 2024. PMID: 39516585 Free PMC article.
-
Differential interference with Pythium ultimum sporangial activation and germination by Enterobacter cloacae in the corn and cucumber spermospheres.Appl Environ Microbiol. 2008 Jul;74(14):4285-91. doi: 10.1128/AEM.00263-08. Epub 2008 May 30. Appl Environ Microbiol. 2008. PMID: 18515482 Free PMC article.
References
-
- Bewley, J. D., and M. Black. 1994. Seeds: physiology of development and germination. Plenum Press, New York, NY.
-
- Clark, D. P., and J. E. Cronan, Jr. 1996. Two-carbon compounds and fatty acids as carbon sources, p. 343-357. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, DC.
-
- DiRusso, C. C., P. N. Black, and J. D. Weimar. 1999. Molecular inroads into the regulation and metabolism of fatty acids: lessons from bacteria. Prog. Lipid Res. 38:129-197. - PubMed
-
- Folch, J., M. Lees, and G. H. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

