Two-log increase in sensitivity for detection of norovirus in complex samples by concentration with porcine gastric mucin conjugated to magnetic beads
- PMID: 18515489
- PMCID: PMC2493163
- DOI: 10.1128/AEM.00539-08
Two-log increase in sensitivity for detection of norovirus in complex samples by concentration with porcine gastric mucin conjugated to magnetic beads
Abstract
Human histo-blood group antigens (HBGA) have been identified previously as candidate receptors for human norovirus (NOR). Type A, type H1, and Lewis HBGA in humans have been identified as major HBGA for NOR binding. We have found that pig stomach (gastric) mucin (PGM) contains blood group A, H1, and Lewis b HBGA and binds to multiple strains of NOR more broadly than do specific antibodies to NOR. Both genogroup I (GGI) and GGII NOR strains were recovered by PGM-conjugated magnetic beads. A fecal sample containing GGII NOR was detected at a dilution of 1:1,000,000 by the standard RNA extraction procedure, whereas NOR in a 1:100,000,000 dilution could be concentrated by PGM-conjugated magnetic beads and NOR in spiked food samples (e.g., oyster extract, strawberry, raspberry, and lettuce) was captured by PGM, thus minimizing the reverse transcription-PCR inhibitors in food and increasing sensitivity.
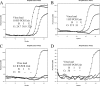
Figures


References
-
- Daniels, N. A., D. A. Bergmire-Sweat, K. J. Schwab, K. A. Hendricks, S. Reddy, S. M. Rowe, R. L. Fankhauser, S. S. Monroe, R. L. Atmar, R. I. Glass, and P. Mead. 2000. A foodborne outbreak of gastroenteritis associated with Norwalk-like viruses: first molecular traceback to deli sandwiches contaminated during preparation. J. Infect. Dis. 181:1467-1470. - PubMed
-
- Gilpatrick, S. G., K. J. Schwab, M. K. Estes, and R. L. Atmar. 2000. Development of an immunomagnetic capture reverse transcription-PCR assay for the detection of Norwalk virus. J. Virol. Methods 90:69-78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

