MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana
- PMID: 18515503
- PMCID: PMC2438462
- DOI: 10.1105/tpc.107.055855
MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana
Abstract
Nitric oxide (NO) and reactive oxygen species (ROS) act as signals in innate immunity in plants. The radical burst is induced by INF1 elicitin, produced by the oomycete pathogen Phytophthora infestans. NO ASSOCIATED1 (NOA1) and NADPH oxidase participate in the radical burst. Here, we show that mitogen-activated protein kinase (MAPK) cascades MEK2-SIPK/NTF4 and MEK1-NTF6 participate in the regulation of the radical burst. NO generation was induced by conditional activation of SIPK/NTF4, but not by NTF6, in Nicotiana benthamiana leaves. INF1- and SIPK/NTF4-mediated NO bursts were compromised by the knockdown of NOA1. However, ROS generation was induced by either SIPK/NTF4 or NTF6. INF1- and MAPK-mediated ROS generation was eliminated by silencing Respiratory Burst Oxidase Homolog B (RBOHB), an inducible form of the NADPH oxidase. INF1-induced expression of RBOHB was compromised in SIPK/NTF4/NTF6-silenced leaves. These results indicated that INF1 regulates NOA1-mediated NO and RBOHB-dependent ROS generation through MAPK cascades. NOA1 silencing induced high susceptibility to Colletotrichum orbiculare but not to P. infestans; conversely, RBOHB silencing decreased resistance to P. infestans but not to C. orbiculare. These results indicate that the effects of the radical burst on the defense response appear to be diverse in plant-pathogen interactions.
Figures
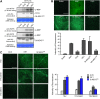
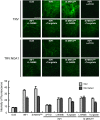





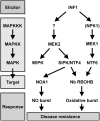
References
-
- Alamillo, J.M., and García-Olmedo, F. (2001). Effects of urate, a natural scavenger of peroxynitrite-mediated toxicity, in the response of Arabidopsis thaliana to the bacterial pathogen Pseudomonas syringae. Plant J. 25 529–540. - PubMed
-
- Bradley, D.J., Kjellbom, P., and Lamb, C.J. (1992). Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell 70 21–30. - PubMed
-
- Bright, J., Desikan, R., Hancock, J.T., Weir, I., and Neill, S.J. (2006). ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45 113–122. - PubMed
-
- Butt, Y.K.-C., Lum, J.H.-K., and Lo, S.C.-L. (2003). Proteomic identification of plant proteins probed by mammalian nitric oxide synthase antibodies. Planta 216 762–771. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

