Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice
- PMID: 18515909
- PMCID: PMC3837456
- DOI: 10.1194/jlr.M800248-JLR200
Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice
Abstract
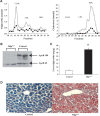
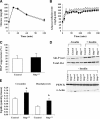
The liver secretes triglyceride-rich VLDLs, and the triglycerides in these particles are taken up by peripheral tissues, mainly heart, skeletal muscle, and adipose tissue. Blocking hepatic VLDL secretion interferes with the delivery of liver-derived triglycerides to peripheral tissues and results in an accumulation of triglycerides in the liver. However, it is unclear how interfering with hepatic triglyceride secretion affects adiposity, muscle triglyceride stores, and insulin sensitivity. To explore these issues, we examined mice that cannot secrete VLDL [due to the absence of microsomal triglyceride transfer protein (Mttp) in the liver]. These mice exhibit markedly reduced levels of apolipoprotein B-100 in the plasma, along with reduced levels of triglycerides in the plasma. Despite the low plasma triglyceride levels, triglyceride levels in skeletal muscle were unaffected. Adiposity and adipose tissue triglyceride synthesis rates were also normal, and body weight curves were unaffected. Even though the blockade of VLDL secretion caused hepatic steatosis accompanied by increased ceramides and diacylglycerols in the liver, the mice exhibited normal glucose tolerance and were sensitive to insulin at the whole-body level, as judged by hyperinsulinemic euglycemic clamp studies. Normal hepatic glucose production and insulin signaling were also maintained in the fatty liver induced by Mttp deletion. Thus, blocking VLDL secretion causes hepatic steatosis without insulin resistance, and there is little effect on muscle triglyceride stores or adiposity.
Figures

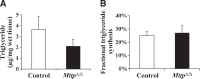
References
-
- White DA, Bennett AJ, Billett MA, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br. J. Nutr. 1998;80:219–229. - PubMed
-
- Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996;37:693–707. - PubMed
-
- Bjorkegren J, Beigneux A, Bergo MO, Maher JJ, Young SG. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J. Biol. Chem. 2002;277:5476–5483. - PubMed
-
- Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann. Med. 2005;37:347–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

