Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency
- PMID: 18516091
- PMCID: PMC2594014
- DOI: 10.1038/ncb1744
Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency
Erratum in
- Nat Cell Biol. 2012 Jun;14(6):649. Pitel, Kevin [corrected to Pitel, Kevin S]
Abstract
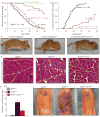
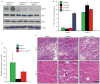
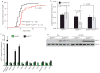
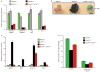
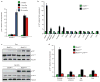
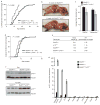
Expression of p16(Ink4a) and p19(Arf) increases with age in both rodent and human tissues. However, whether these tumour suppressors are effectors of ageing remains unclear, mainly because knockout mice lacking p16(Ink4a) or p19(Arf) die early of tumours. Here, we show that skeletal muscle and fat, two tissues that develop early ageing-associated phenotypes in response to BubR1 insufficiency, have high levels of p16(Ink4a) and p19(Arf). Inactivation of p16(Ink4a) in BubR1-insufficient mice attenuates both cellular senescence and premature ageing in these tissues. Conversely, p19(Arf) inactivation exacerbates senescence and ageing in BubR1 mutant mice. Thus, we identify BubR1 insufficiency as a trigger for activation of the Cdkn2a locus in certain mouse tissues, and demonstrate that p16(Ink4a) is an effector and p19(Arf) an attenuator of senescence and ageing in these tissues.
Figures


References
-
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. - PubMed
-
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Rev Mol Cell Biol. 2007;8:729–740. - PubMed
-
- Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. - PubMed
-
- Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. - PubMed
-
- Vousden KH, Lane D. P p53 in health and disease. Nature Rev Mol Cell Biol. 2007;8:275–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

