Human metapneumovirus glycoprotein G inhibits innate immune responses
- PMID: 18516301
- PMCID: PMC2386556
- DOI: 10.1371/journal.ppat.1000077
Human metapneumovirus glycoprotein G inhibits innate immune responses
Abstract
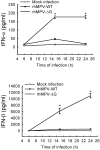
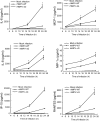
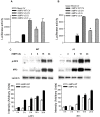
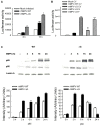
Human metapneumovirus (hMPV) is a leading cause of acute respiratory tract infection in infants, as well as in the elderly and immunocompromised patients. No effective treatment or vaccine for hMPV is currently available. A recombinant hMPV lacking the G protein (rhMPV-Delta G) was recently developed as a potential vaccine candidate and shown to be attenuated in the respiratory tract of a rodent model of infection. The mechanism of its attenuation, as well as the role of G protein in modulation of hMPV-induced cellular responses in vitro, as well as in vivo, is currently unknown. In this study, we found that rhMPV-Delta G-infected airway epithelial cells produced higher levels of chemokines and type I interferon (IFN) compared to cells infected with rhMPV-WT. Infection of airway epithelial cells with rhMPV-Delta G enhanced activation of transcription factors belonging to the nuclear factor (NF)-kappaB and interferon regulatory factor (IRF) families, as revealed by increased nuclear translocation and/or phosphorylation of these transcription factors. Compared to rhMPV-WT, rhMPV-Delta G also increased IRF- and NF-kappaB-dependent gene transcription, which was reversely inhibited by G protein expression. Since RNA helicases have been shown to play a fundamental role in initiating viral-induced cellular signaling, we investigated whether retinoic induced gene (RIG)-I was the target of G protein inhibitory activity. We found that indeed G protein associated with RIG-I and inhibited RIG-I-dependent gene transcription, identifying an important mechanism by which hMPV affects innate immune responses. This is the first study investigating the role of hMPV G protein in cellular signaling and identifies G as an important virulence factor, as it inhibits the production of important immune and antiviral mediators by targeting RIG-I, a major intracellular viral RNA sensor.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. - PubMed
-
- van den Hoogen BG, Bestebroer TM, Osterhaus AD, Fouchier RA. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–132. - PubMed
-
- Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, et al. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J Virol. 2005;79:12608–12613. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

