Protein dynamics explain the allosteric behaviors of hemoglobin
- PMID: 18519045
- PMCID: PMC2668241
- DOI: 10.1016/j.bbapap.2008.04.025
Protein dynamics explain the allosteric behaviors of hemoglobin
Abstract
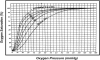
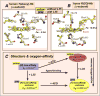
Bohr, Hasselbalch, and Krogh discovered homotropic and heterotropic allosteric behaviors of hemoglobin (Hb) in 1903/1904. A chronological description since then of selected principal models of the allosteric mechanism of Hb, such as the Adair scheme, the MWC two-state concerted model, the KNF induced-fit sequential model, the Perutz stereochemical model, the tertiary two-state model, and the global allostery model (an expanded MWC models), is concisely presented, followed by analysis and discussion of their limitations and deficiencies. The determination of X-ray crystallographic structures of deoxy- and ligated-Hb and the structure-based stereochemical model by Perutz are an epoch-making event in this history. However, his assignment of low-affinity deoxy- and high-affinity oxy-quaternary structures of Hb to the T- and R-states, respectively, though apparently reasonable, and as well as his hypothesis that the T-/R-quaternary structural transition regulates the oxygen-affinity, have created confusions and side-tracked studies of Hb on the structure-function relationship. The differences in static molecular structures of Hb between T(deoxy)- and R(oxy)-quaternary states reported in detail by Perutz and others are ligation-linked structural changes, but not related to the control/regulation of the oxygen-affinity. The oxygen-affinity (K(T) and K(R)) of Hb has been shown to be regulated by the heterotropic effector-linked tertiary structural changes without involving the T/R-quaternary changes. However, a recent high-resolution crystallographic analysis of Hb with different oxygen-affinities shows that static molecular structures of Hb determined by crystallography can neither identify the nature of the T(low-affinity) functional state nor decipher the mechanism by which Hb stores free energy in the T(low-affinity) functional state. Molecular dynamics simulations show that fluctuations of helices of oxy-Hb are increased upon de-oxygenation and/or binding 2,3-biphosphoglycerate. These are known to lower the oxygen-affinity of Hb. It is proposed that the coordination mode of the heme Fe with proximal and distal His is modulated by these helical fluctuations, resulting in the modulation of the oxygen-affinity of Hb. Therefore, it is proposed that the oxygen-affinity of Hb is regulated by pentanary (the 5th-order time-dependent or dynamic) tertiary structural changes rather than the T-/R-quaternary structural transitions in Hb. Homotropic and heterotropic allosteric effects of Hb are oxygen- and effector-linked, conformational entropy-driven entropy-enthalpy compensation phenomena and not much to do with static structural changes. The dynamic allostery model, which integrates these observations, provides the structural basis for the global allostery model (an expanded MWC model).
Figures







References
-
- Bohr C. Theoretische Behandlung der quantitativen Verhaltnis bei der Sauerstoffaufnahme des Hamoglobin. Zentr Physiol. 1903;17:682–689.
-
- Bohr C, Hasselbalch KA, Krogh A. Ueber einen in biologischer Beziehung wichtigen Einfluss den die Kohlen-sauerspannung des Blutes aufdessen Sauerstoffbindung ubt. Skand Arch Physiol. 1904;15:401–412.
-
- Monod J, Jacob EF. General conclusion: Teleonomic mechanism in cellular metabolism, growth, and differentiation. Cold Spring Harbor Sym Quant Biol. 1961;26:389–401. - PubMed
-
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. - PubMed
-
- Adair GS. A critical study of the direct method of measuring the osmotic pressure of haemoglobin. Proc Roy Soc (London) Ser A. 1925;108A:627–637.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

