Remodelling of gap junctions and connexin expression in diseased myocardium
- PMID: 18519446
- PMCID: PMC2533424
- DOI: 10.1093/cvr/cvn133
Remodelling of gap junctions and connexin expression in diseased myocardium
Abstract
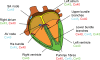
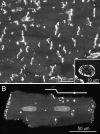
Gap junctions form the cell-to-cell pathways for propagation of the precisely orchestrated patterns of current flow that govern the regular rhythm of the healthy heart. As in most tissues and organs, multiple connexin types are expressed in the heart: connexin43 (Cx43), Cx40 and Cx45 are found in distinctive combinations and relative quantities in different, functionally-specialized subsets of cardiac myocyte. Mutations in genes that encode connexins have only rarely been identified as being a cause of human cardiac disease, but remodelling of connexin expression and gap junction organization are well documented in acquired adult heart disease, notably ischaemic heart disease and heart failure. Remodelling may take the form of alterations in (i) the distribution of gap junctions and (ii) the amount and type of connexins expressed. Heterogeneous reduction in Cx43 expression and disordering in gap junction distribution feature in human ventricular disease and correlate with electrophysiologically identified arrhythmic changes and contractile dysfunction in animal models. Disease-related alterations in Cx45 and Cx40 expression have also been reported, and some of the functional implications of these are beginning to emerge. Apart from ventricular disease, various features of gap junction organization and connexin expression have been implicated in the initiation and persistence of the most common form of atrial arrhythmia, atrial fibrillation, though the disparate findings in this area remain to be clarified. Other major tasks ahead focus on the Purkinje/working ventricular myocyte interface and its role in normal and abnormal impulse propagation, connexin-interacting proteins and their regulatory functions, and on defining the precise functional properties conferred by the distinctive connexin co-expression patterns of different myocyte types in health and disease.
Figures



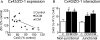
References
-
- Harris AL, Locke D. Connexins: A Guide. Secaucus: Humana Press - Springer; 2008.
-
- Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62:368–377. - PubMed
-
- Severs NJ, Dupont E, Thomas N, Kaba R, Rothery S, Jain R, et al. Alterations in cardiac connexin expression in cardiomyopathies. Adv Cardiol. 2006;42:228–242. - PubMed
-
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. - PubMed
-
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

