Distinct functions of POT1 at telomeres
- PMID: 18519588
- PMCID: PMC2519730
- DOI: 10.1128/MCB.00048-08
Distinct functions of POT1 at telomeres
Abstract
The mammalian protein POT1 binds to telomeric single-stranded DNA (ssDNA), protecting chromosome ends from being detected as sites of DNA damage. POT1 is composed of an N-terminal ssDNA-binding domain and a C-terminal protein interaction domain. With regard to the latter, POT1 heterodimerizes with the protein TPP1 to foster binding to telomeric ssDNA in vitro and binds the telomeric double-stranded-DNA-binding protein TRF2. We sought to determine which of these functions-ssDNA, TPP1, or TRF2 binding-was required to protect chromosome ends from being detected as DNA damage. Using separation-of-function POT1 mutants deficient in one of these three activities, we found that binding to TRF2 is dispensable for protecting telomeres but fosters robust loading of POT1 onto telomeric chromatin. Furthermore, we found that the telomeric ssDNA-binding activity and binding to TPP1 are required in cis for POT1 to protect telomeres. Mechanistically, binding of POT1 to telomeric ssDNA and association with TPP1 inhibit the localization of RPA, which can function as a DNA damage sensor, to telomeres.
Figures
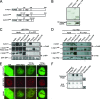

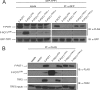
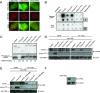
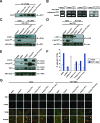

References
-
- Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2921171-1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
