Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging
- PMID: 18519635
- PMCID: PMC2732416
- DOI: 10.1101/gad.1657108
Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging
Abstract
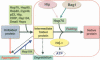
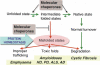
The long-term health of the cell is inextricably linked to protein quality control. Under optimal conditions this is accomplished by protein homeostasis, a highly complex network of molecular interactions that balances protein biosynthesis, folding, translocation, assembly/disassembly, and clearance. This review will examine the consequences of an imbalance in homeostasis on the flux of misfolded proteins that, if unattended, can result in severe molecular damage to the cell. Adaptation and survival requires the ability to sense damaged proteins and to coordinate the activities of protective stress response pathways and chaperone networks. Yet, despite the abundance and apparent capacity of chaperones and other components of homeostasis to restore folding equilibrium, the cell appears poorly adapted for chronic proteotoxic stress when conformationally challenged aggregation-prone proteins are expressed in cancer, metabolic disease, and neurodegenerative disease. The decline in biosynthetic and repair activities that compromises the integrity of the proteome is influenced strongly by genes that control aging, thus linking stress and protein homeostasis with the health and life span of the organism.
Figures



References
-
- Abravaya K., Phillips B., Morimoto R.I. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes & Dev. 1991;5:2117–2127. - PubMed
-
- Abravaya K., Myers M.P., Murphy S.P., Morimoto R.I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes & Dev. 1992;6:1153–1164. - PubMed
-
- Ananthan J., Goldberg A.L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–524. - PubMed
-
- Anckar J., Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv. Exp. Med. Biol. 2007;594:78–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
