Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas
- PMID: 18519637
- PMCID: PMC2418581
- DOI: 10.1101/gad.1663208
Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas
Abstract
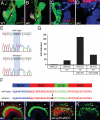
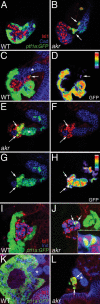
The mechanisms regulating pancreatic endocrine versus exocrine fate are not well defined. By analyzing the effects of Ptf1a partial loss of function, we uncovered novel roles for this transcription factor in determining pancreatic fates. In a newly identified hypomorphic ptf1a mutant, pancreatic cells that would normally express ptf1a and become exocrine cells, express the endocrine marker Isl1, indicating a cell fate switch. Surprisingly, a milder reduction of Ptf1a leads to an even greater increase of ectopic endocrine cells, suggesting that Ptf1a also plays a role in promoting endocrine development. We propose that low levels of Ptf1a promote endocrine fate, whereas high levels repress endocrine fate and promote exocrine fate.
Figures


References
-
- Ahlgren U., Pfaff S.L., Jessell T.M., Edlund T., Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. - PubMed
-
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D.J., Honjo T., de Hrabe Angelis M., Lendahl U., Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. - PubMed
-
- Bertuzzi F., Marzorati S., Secchi A. Islet cell transplantation. Curr. Mol. Med. 2006;6:369–374. - PubMed
-
- Chiang M.K., Melton D.A. Single-cell transcript analysis of pancreas development. Dev. Cell. 2003;4:383–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
