TopBP1 activates ATR through ATRIP and a PIKK regulatory domain
- PMID: 18519640
- PMCID: PMC2418584
- DOI: 10.1101/gad.1666208
TopBP1 activates ATR through ATRIP and a PIKK regulatory domain
Abstract
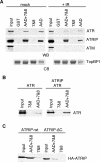
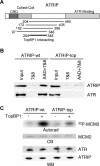
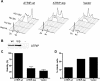
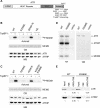
The ATR (ATM and Rad3-related) kinase and its regulatory partner ATRIP (ATR-interacting protein) coordinate checkpoint responses to DNA damage and replication stress. TopBP1 functions as a general activator of ATR. However, the mechanism by which TopBP1 activates ATR is unknown. Here, we show that ATRIP contains a TopBP1-interacting region that is necessary for the association of TopBP1 and ATR, for TopBP1-mediated activation of ATR, and for cells to survive and recover DNA synthesis following replication stress. We demonstrate that this region is functionally conserved in the Saccharomyces cerevisiae ATRIP ortholog Ddc2, suggesting a conserved mechanism of regulation. In addition, we identify a domain of ATR that is critical for its activation by TopBP1. Mutations of the ATR PRD (PIKK [phosphoinositide 3-kinase related kinase] Regulatory Domain) do not affect the basal kinase activity of ATR but prevent its activation. Cellular complementation experiments demonstrate that TopBP1-mediated ATR activation is required for checkpoint signaling and cellular viability. The PRDs of ATM and mTOR (mammalian target of rapamycin) were shown previously to regulate the activities of these kinases, and our data indicate that the DNA-PKcs (DNA-dependent protein kinase catalytic subunit) PRD is important for DNA-PKcs regulation. Therefore, divergent amino acid sequences within the PRD and a unique protein partner allow each of these PIK kinases to respond to distinct cellular events.
Figures

Comment in
-
How ATR turns on: TopBP1 goes on ATRIP with ATR.Genes Dev. 2008 Jun 1;22(11):1416-21. doi: 10.1101/gad.1685108. Genes Dev. 2008. PMID: 18519633 Free PMC article.
References
-
- Abraham R.T. PI 3-kinase related kinases: ‘Big’ players in stress-induced signaling pathways. DNA Repair (Amst.) 2004;3:883–887. - PubMed
-
- Banin S., Moyal L., Shieh S., Taya Y., Anderson C.W., Chessa L., Smorodinsky N.I., Prives C., Reiss Y., Shiloh Y., et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
