Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation
- PMID: 18519646
- PMCID: PMC2413039
- DOI: 10.1084/jem.20070846
Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation
Abstract
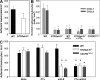
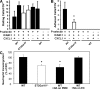
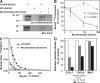
Recent in vitro studies have suggested a role for sialylation in chemokine receptor binding to its ligand (Bannert, N., S. Craig, M. Farzan, D. Sogah, N.V. Santo, H. Choe, and J. Sodroski. 2001. J. Exp. Med. 194:1661-1673). This prompted us to investigate chemokine-induced leukocyte adhesion in inflamed cremaster muscle venules of alpha2,3 sialyltransferase (ST3Gal-IV)-deficient mice. We found a marked reduction in leukocyte adhesion to inflamed microvessels upon injection of the CXCR2 ligands CXCL1 (keratinocyte-derived chemokine) or CXCL8 (interleukin 8). In addition, extravasation of ST3Gal-IV(-/-) neutrophils into thioglycollate-pretreated peritoneal cavities was significantly decreased. In vitro assays revealed that CXCL8 binding to isolated ST3Gal-IV(-/-) neutrophils was markedly impaired. Furthermore, CXCL1-mediated adhesion of ST3Gal-IV(-/-) leukocytes at physiological flow conditions, as well as transendothelial migration of ST3Gal-IV(-/-) leukocytes in response to CXCL1, was significantly reduced. In human neutrophils, enzymatic desialylation decreased binding of CXCR2 ligands to the neutrophil surface and diminished neutrophil degranulation in response to these chemokines. In addition, binding of alpha2,3-linked sialic acid-specific Maackia amurensis lectin II to purified CXCR2 from neuraminidase-treated CXCR2-transfected HEK293 cells was markedly impaired. Collectively, we provide substantial evidence that sialylation by ST3Gal-IV significantly contributes to CXCR2-mediated leukocyte adhesion during inflammation in vivo.
Figures



References
-
- Springer, T.A. 1995. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57:827–872. - PubMed
-
- Vestweber, D., and J.E. Blanks. 1999. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 79:181–213. - PubMed
-
- Laudanna, C., and R. Alon. 2006. Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb. Haemost. 95:5–11. - PubMed
-
- Rainger, G.E., A.C. Fisher, and G.B. Nash. 1997. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am. J. Physiol. 272:H114–H122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

