A unique B2 B cell subset in the intestine
- PMID: 18519649
- PMCID: PMC2413032
- DOI: 10.1084/jem.20071572
A unique B2 B cell subset in the intestine
Abstract
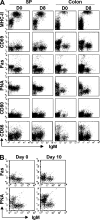
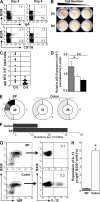
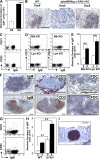
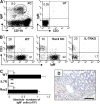
Over 80% of the body's activated B cells are located in mucosal sites, including the intestine. The intestine contains IgM(+) B cells, but these cells have not been characterized phenotypically or in terms of their developmental origins. We describe a previously unidentified and unique subset of immunoglobulin M(+) B cells that present with an AA4.1(-)CD21(-)CD23(-) major histocompatibility complex class II(bright) surface phenotype and are characterized by a low frequency of somatic hypermutation and the potential ability to produce interleukin-12p70. This B cell subset resides within the normal mucosa of the large intestine and expands in response to inflammation. Some of these intestinal B cells originate from the AA4.1(+) immature B2 cell pool in the steady state and are also recruited from the recirculating naive B cell pool in the context of intestinal inflammation. They develop in an antigen-independent and BAFF-dependent manner in the absence of T cell help. Expansion of these cells can be induced in the absence of the spleen and gut-associated lymphoid tissues. These results describe the existence of an alternative pathway of B cell maturation in the periphery that gives rise to a tissue-specific B cell subset.
Figures



Comment in
-
Sheepish B cells: evidence for antigen-independent antibody diversification in humans and mice.J Exp Med. 2008 Jun 9;205(6):1251-4. doi: 10.1084/jem.20081057. Epub 2008 Jun 2. J Exp Med. 2008. PMID: 18519651 Free PMC article.
References
-
- Hardy, R.R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621. - PubMed
-
- Su, T.T., B. Guo, B. Wei, J. Braun, and D. Rawlings. 2004. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol. Rev. 197:161–178. - PubMed
-
- Allman, D., B. Srivastava, and R.C. Lindsley. 2004. Alternative routes to maturity: branch points and pathways for generating follicular and marginal zone B cells. Immunol. Rev. 197:147–160. - PubMed
-
- Cancro, M.P. 2004. Peripheral B-cell maturation: the intersection of selection and homeostasis. Immunol. Rev. 197:89–101. - PubMed
-
- Pillai, S., A. Cariappa, and S. Moran. 2005. Marginal zone B cells. Annu. Rev. Immunol. 23:161–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

