Sheepish B cells: evidence for antigen-independent antibody diversification in humans and mice
- PMID: 18519651
- PMCID: PMC2413022
- DOI: 10.1084/jem.20081057
Sheepish B cells: evidence for antigen-independent antibody diversification in humans and mice
Abstract
Antibody diversity is first generated by rearrangement of immunoglobulin (Ig) genes during B cell development in the bone marrow, and later by antigen-driven diversification in germinal centers (GCs). New data in humans and mice now identify specific B cell populations that may have undergone antigen-independent hypermutation outside GCs.
Figures
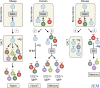
Comment on
-
Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants.J Exp Med. 2008 Jun 9;205(6):1331-42. doi: 10.1084/jem.20071555. Epub 2008 Jun 2. J Exp Med. 2008. PMID: 18519648 Free PMC article.
-
A unique B2 B cell subset in the intestine.J Exp Med. 2008 Jun 9;205(6):1343-55. doi: 10.1084/jem.20071572. Epub 2008 Jun 2. J Exp Med. 2008. PMID: 18519649 Free PMC article.
References
-
- Neuberger, M.S., J.M. Di Noia, R.C. Beale, G.T. Williams, Z. Yang, and C. Rada. 2005. Somatic hypermutation at A.T pairs: polymerase error versus dUTP incorporation. Nat. Rev. Immunol. 5:171–178. - PubMed
-
- Weller, S., M.C. Braun, B.K. Tan, A. Rosenwald, C. Cordier, M.E. Conley, A. Plebani, D.S. Kumararatne, D. Bonnet, O. Tournilhac, et al. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 104:3647–3654. - PMC - PubMed

