Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells
- PMID: 18519693
- PMCID: PMC2725025
- DOI: 10.1158/0008-5472.CAN-08-0943
Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells
Abstract
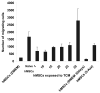
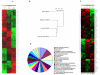
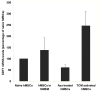
Carcinoma-associated fibroblasts (CAF) have recently been implicated in important aspects of epithelial solid tumor biology, such as neoplastic progression, tumor growth, angiogenesis, and metastasis. However, neither the source of CAFs nor the differences between CAFs and fibroblasts from nonneoplastic tissue have been well defined. In this study, we show that human bone marrow-derived mesenchymal stem cells (hMSCs) exposed to tumor-conditioned medium (TCM) over a prolonged period of time assume a CAF-like myofibroblastic phenotype. More importantly, these cells exhibit functional properties of CAFs, including sustained expression of stromal-derived factor-1 (SDF-1) and the ability to promote tumor cell growth both in vitro and in an in vivo coimplantation model, and expression of myofibroblast markers, including alpha-smooth muscle actin and fibroblast surface protein. hMSCs induced to differentiate to a myofibroblast-like phenotype using 5-azacytidine do not promote tumor cell growth as efficiently as hMSCs cultured in TCM nor do they show increased SDF-1 expression. Furthermore, gene expression profiling revealed similarities between TCM-exposed hMSCs and CAFs. Taken together, these data suggest that hMSCs are a source of CAFs and can be used in the modeling of tumor-stroma interactions. To our knowledge, this is the first report showing that hMSCs become activated and resemble carcinoma-associated myofibroblasts on prolonged exposure to conditioned medium from MDAMB231 human breast cancer cells.
Figures





Similar articles
-
Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors.Cell Oncol (Dordr). 2011 Feb;34(1):55-67. doi: 10.1007/s13402-011-0012-1. Epub 2011 Feb 15. Cell Oncol (Dordr). 2011. PMID: 21327615
-
GRP78 secreted by tumor cells stimulates differentiation of bone marrow mesenchymal stem cells to cancer-associated fibroblasts.Biochem Biophys Res Commun. 2013 Nov 1;440(4):558-63. doi: 10.1016/j.bbrc.2013.09.108. Epub 2013 Oct 7. Biochem Biophys Res Commun. 2013. PMID: 24113381
-
Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors.Anal Cell Pathol (Amst). 2010;33(2):61-79. doi: 10.3233/ACP-CLO-2010-0535. Anal Cell Pathol (Amst). 2010. PMID: 20978328 Free PMC article.
-
Mesenchymal stem cells: flip side of the coin.Cancer Res. 2009 Feb 15;69(4):1255-8. doi: 10.1158/0008-5472.CAN-08-3562. Epub 2009 Feb 10. Cancer Res. 2009. PMID: 19208837 Review.
-
Hematopoietic stem cell derived carcinoma-associated fibroblasts: a novel origin.Int J Clin Exp Pathol. 2012;5(9):863-73. Epub 2012 Oct 20. Int J Clin Exp Pathol. 2012. PMID: 23119103 Free PMC article. Review.
Cited by
-
Further Understanding of the Immune Microenvironment in Head and Neck Squamous Cell Carcinoma: Implications for Prognosis.Cancer Manag Res. 2021 May 17;13:3973-3980. doi: 10.2147/CMAR.S277907. eCollection 2021. Cancer Manag Res. 2021. PMID: 34040438 Free PMC article. Review.
-
Fibroblasts direct differentiation of human breast epithelial progenitors.Breast Cancer Res. 2020 Sep 29;22(1):102. doi: 10.1186/s13058-020-01344-0. Breast Cancer Res. 2020. PMID: 32993755 Free PMC article.
-
Modulation of the antitumor immune response by cancer-associated fibroblasts: mechanisms and targeting strategies to hamper their immunosuppressive functions.Explor Target Antitumor Ther. 2022;3(5):598-629. doi: 10.37349/etat.2022.00103. Epub 2022 Oct 27. Explor Target Antitumor Ther. 2022. PMID: 36338519 Free PMC article. Review.
-
Bone marrow cells as precursors of the tumor stroma.Exp Cell Res. 2013 Jul 1;319(11):1650-6. doi: 10.1016/j.yexcr.2013.03.006. Epub 2013 Mar 13. Exp Cell Res. 2013. PMID: 23499739 Free PMC article. Review.
-
Identification of cancer stem cells provides novel tumor models for drug discovery.Front Med. 2012 Jun;6(2):112-21. doi: 10.1007/s11684-012-0199-1. Epub 2012 May 9. Front Med. 2012. PMID: 22573222 Review.
References
-
- Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol. 2005;17:180–6. - PubMed
-
- Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 1997;39:174–7. - PubMed
-
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. - PubMed
-
- Ishii G, Sangai T, Oda T, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

