A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer
- PMID: 18519778
- PMCID: PMC8603216
- DOI: 10.1158/1078-0432.CCR-07-4740
A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer
Abstract
Purpose: Interleukin-18 (IL-18) is an immunostimulatory cytokine with antitumor activity in preclinical models. A phase I study of recombinant human IL-18 (rhIL-18) was done to determine the toxicity, pharmacokinetics, and biological activities of rhIL-18 administered at different doses in two different schedules to patients with advanced cancer.
Experimental design: Cohorts of three to four patients were given escalating doses of rhIL-18 as a 2-h i.v. infusion either on 5 consecutive days repeated every 28 days (group A) or once a week (group B) for up to 6 months. Toxicities were graded using standard criteria. Blood samples were obtained for safety, pharmacokinetic, and pharmacodynamic measurements.
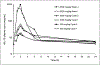
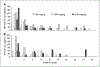
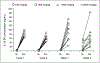
Results: Nineteen patients (10 melanoma and 9 renal cell cancer) were given rhIL-18 in doses of 100, 500, or 1,000 microg/kg (group A) or 100, 1,000, or 2,000 microg/kg (group B). Common side effects included chills, fever, headache, fatigue, and nausea. Common laboratory abnormalities included transient, asymptomatic grade 1 to 3 lymphopenia, grade 1 to 4 hyperglycemia, grade 1 to 2 anemia, neutropenia, hypoalbuminemia, liver enzyme elevations, and serum creatinine elevations. No dose-limiting toxicities were observed. Biological effects of rhIL-18 included transient lymphopenia and increased expression of activation antigens on lymphocytes. Increases in serum concentrations of IFN-gamma, granulocyte macrophage colony-stimulating factor, and IL-18-binding protein were observed following dosing.
Conclusions: rhIL-18 can be given in biologically active doses by either weekly infusions or daily infusions for 5 days repeated every 28 days to patients with advanced cancer. Toxicity was generally mild to moderate, and a maximum tolerated dose of rhIL-18 by either schedule was not determined.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
K. Koch, L. Kirby, W. Bell, and M. Dar are employed by GlaxoSmithKline. M. Robertson received a research grant from GlaxoSmithKline and has a financial interest in Eli Lilly Corporation. The other authors have disclosed no potential conflicts of interest.
Figures

References
-
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001;19:423–74. - PubMed
-
- Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol 2003;73:213–24. - PubMed
-
- Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol 2002;14:117–22. - PubMed
-
- Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 1995;378:88–91. - PubMed
-
- Robinson D, Shibuya K, Mui A, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity 1997;7:571–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

