Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart
- PMID: 18519848
- PMCID: PMC2679251
- DOI: 10.1161/CIRCULATIONAHA.108.767673
Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart
Abstract
Background: The Akt protein kinase is an important mediator of cardiac myocyte growth and survival. To identify factors with novel therapeutic applications in cardiac diseases, we focused on the identification of factors secreted from Akt1-activated cells that have cardioprotective effects through autocrine/paracrine mechanisms.
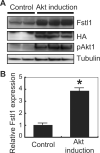
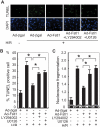
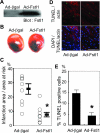
Methods and results: Using an inducible Akt1 transgenic mouse model, we have found that follistatin-like 1 (Fstl1) protein and transcript expression are increased 4.0- and 2.0-fold, respectively, by Akt activation in the heart (P<0.05). Fstl1 transcript was also upregulated in response to myocardial stresses including transverse aortic constriction, ischemia/reperfusion injury, and myocardial infarction. Adenovirus-mediated overexpression of Fstl1 protected cultured neonatal rat ventricular myocytes from hypoxia/reoxygenation-induced apoptosis (P<0.01), and this protective effect was dependent on the upregulation of both Akt and ERK activities. Conversely, knockdown of Fstl1 in cardiac myocytes decreased basal Akt signaling and increased the frequency of apoptotic death in vitro (P<0.01). The intravenous administration of an adenoviral encoding Fstl1 to mice resulted in a 66.0% reduction in myocardial infarct size after ischemia/reperfusion injury that was accompanied by a 70.9% reduction in apoptosis in the heart (P<0.01).
Conclusions: These results indicate that Fstl1 is a cardiac-secreted factor that functions as an antiapoptotic protein. Fstl1 could play a role in myocardial maintenance and repair in response to harmful stimuli.
Figures





References
-
- Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. - PubMed
-
- Miao W, Luo Z, Kitsis RN, Walsh K. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemiareperfusion injury in vivo. J Mol Cell Cardiol. 2000;32:2397–2402. - PubMed
-
- Taniyama Y, Walsh K. Elevated myocardial Akt signaling ameliorates doxorubicin-induced congestive heart failure and promotes heart growth. J Mol Cell Cardiol. 2002;34:1241–1247. - PubMed
-
- Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

