A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results
- PMID: 18520981
- PMCID: PMC4372145
- DOI: 10.1097/WNF.0b013e3181342f32
A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results
Abstract
Objective: To report an 18-month follow-up on creatine and minocycline futility study, the Neuroprotective Exploratory Trials in Parkinson Disease, Futility Study 1 (NET-PD FS-1).
Background: The NET-PD FS-1 futility study on creatine and minocycline found neither agent futile in slowing down the progression of disability in Parkinson disease (PD) at 12 months using the prespecified futility threshold. An additional 6 months of follow-up aimed to assess safety and potential interactions of the study interventions with antiparkinsonian therapy.
Methods: Additional 6 months of follow-up in randomized, blinded phase II trial of creatine (dosage, 10 g/d) and minocycline (dosage, 200 mg/d) in subjects with early PD.
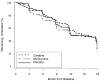
Results: By 18 months, symptomatic treatment of PD symptoms was required in 61% of creatine, 62% of minocycline, and 60% of placebo-treated subjects. Study treatment was prematurely discontinued in 9%, 23%, and 6% of subjects in the creatine, minocycline, and placebo arms, respectively. Creatine and minocycline did not seem to adversely influence the response to symptomatic therapy nor increase adverse events.
Conclusions: Data from this small, 18-month phase II trial of creatine and minocycline do not demonstrate safety concerns that would preclude a large, phase III efficacy trial, although the decreased tolerability of minocycline is a concern.
Figures


References
-
- Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA. 2004;291:358–364. - PubMed
-
- Huse DM, Schulman K, Orsini L, et al. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20:1449–1454. - PubMed
-
- Noyes K, Liu H, Li Y, et al. Economic burden associated with Parkinson’s disease on elderly Medicare beneficiaries. Mov Disord. 2006;21:362–372. - PubMed
-
- The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. - PubMed
-
- Tilley BC, Palesch YY, Kieburtz K, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66:628–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

