Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture
- PMID: 18521572
- PMCID: PMC4264523
- DOI: 10.1007/s00203-008-0387-1
Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture
Abstract
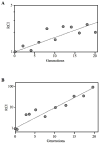
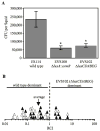
Production of bioluminescence theoretically represents a cost, energetic or otherwise, that could slow Vibrio fischeri growth; however, bioluminescence is also thought to enable full symbiotic colonization of the Euprymna scolopes light organ by V. fischeri. Previous tests of these models have proven inconclusive, partly because they compared nonisogenic strains, or undefined and/or pleiotropic mutants. To test the influence of the bioluminescence-producing lux operon on growth and symbiotic competence, we generated dark luxCDABEG mutants in strains MJ1 and ES114 without disrupting the luxR-luxI regulatory circuit. The MJ1 luxCDABEG mutant out-competed its visibly luminescent parent approximately 26% per generation in a carbon-limited chemostat. Similarly, induction of luminescence in the otherwise dim ES114 strain slowed growth relative to DeltaluxCDABEG mutants. Some culture conditions yielded no detectable effect of luminescence on growth, indicating that luminescence is not always growth limiting; however, luminescence was never found to confer an advantage in culture. In contrast to this conditional disadvantage of lux expression, ES114 achieved approximately fourfold higher populations than its luxCDABEG mutant in the light organ of E. scolopes. These results demonstrate that induction of luxCDABEG can slow V. fischeri growth under certain culture conditions and is a positive symbiotic colonization factor.
Figures

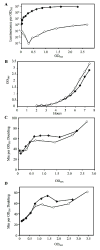
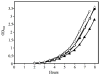
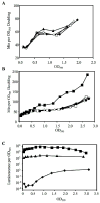
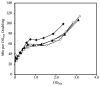
References
-
- Blanc-Potard A, Gari E, Spirito F, Figueroa-Bossi N, Bossi L. RNA polymerase (rpoB) mutants selected for increased resistance to gyrase inhibitors in Salmonella typhimurium. Molec Gen Genet. 1995;247:680–692. - PubMed
-
- Bose JL, et al. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol Microbiol. 2007;65:538–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

