Synthesis of indolines via a domino Cu-catalyzed amidation/cyclization reaction
- PMID: 18522394
- PMCID: PMC2489202
- DOI: 10.1021/ol8008792
Synthesis of indolines via a domino Cu-catalyzed amidation/cyclization reaction
Abstract
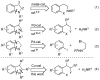
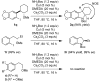
A highly efficient one-pot procedure for the synthesis of indolines and their homologues based on a domino Cu-catalyzed amidation/nucleophilic substitution reaction has been developed. Substituted 2-iodophenethyl mesylates and related compounds afforded the corresponding products in excellent yields. No erosion of optical purity was observed when transforming enantiomerically pure mesylates under the reaction conditions.
Figures

References
-
-
For recent examples of indoline syntheses based on non-metal-catalyzed or radical processes, see: Nicolaou KC, Roecker AJ, Pfefferkorn JA, Cao GQ. J Am Chem Soc. 2000;122:2966.Sanz Gil G, Groth UM. J Am Chem Soc. 2000;122:6789.Dunetz JR, Danheiser RL. J Am Chem Soc. 2005;127:5776.Correa A, Tellitu I, Domínguez E, SanMartin R. J Org Chem. 2006;71:8316.Fuwa H, Sasaki M. Org Lett. 2007;9:3347.Wang Z, Wan W, Jiang H, Hao J. J Org Chem. 2007;72:9364.Gilmore CD, Allan KM, Stoltz BM. J Am Chem Soc. 2008;130:1558.Viswanathan R, Smith CR, Prabhakaran EN, Johnston JN. J Org Chem. 2008;73:3040.Clive DLJ, Peng J, Fletcher SP, Ziffle VE, Wingert D. J Org Chem. 2008;73:2330.
-
-
-
For selected examples, see: Boger DL, Boyce CW, Garbaccio RM, Goldberg JA. Chem Rev. 1997;97:787.Sunazuka T, Hirose T, Shirahata T, Harigaya Y, Hayashi M, Komiyama K, Omura S, Smith AB., III J Am Chem Soc. 2000;122:2122.Dounay AB, Overman LE, Wrobleski AD. J Am Chem Soc. 2005;127:10186.
-
-
-
For selected examples, see: Gruenfeld N, Stanton JL, Yuan AM, Ebetino FH, Browne LJ, Gude C, Huebner CF. J Med Chem. 1983;26:1277.Bromidge SM, Duckworth M, Forbes IT, Ham P, King FD, Thewlis KM, Blaney FE, Naylor CB, Blackburn TP, Kennett GA, Wood MD, Clarke SE. J Med Chem. 1997;40:3494.Hobson LA, Nugent WA, Anderson SR, Deshmukh SS, Haley JJ, III, Liu P, Magnus NA, Sheeran P, Sherbine JP, Stone BRP, Zhu J. Org Process Res Dev. 2007;11:985.
-
-
- Kuang D, Uchida S, Humphry-Baker R, Zakeeruddin SM, Grätzel M. Angew Chem Int Ed. 2008;47:1923. - PubMed
-
- Guram AS, Rennels RA, Buchwald SL. Angew Chem, Int Ed Engl. 1995;34:1348.
- Wolfe JP, Rennels RA, Buchwald SL. Tetrahedron. 1996;52:7525.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

