Synthesis of 5-fluoroalkylated pyrimidine nucleosides via Negishi cross-coupling
- PMID: 18522415
- PMCID: PMC4122541
- DOI: 10.1021/jo800444y
Synthesis of 5-fluoroalkylated pyrimidine nucleosides via Negishi cross-coupling
Abstract
5-Fluoroalkylated pyrimidine nucleosides (1) have potential as therapeutic agents and molecular imaging agents targeting HSV1-tk suicide gene therapy. Thus, straightforward preparation of 5-fluoroalkylated nucleoside derivatives has been developed. Reported herein are the first examples of Pd-catalyzed Negishi cross-coupling of 3-N-benzoyl-3',5'-di-O-benzoyl-5-iodo-2'-deoxyuridine (2a) and 3-N-benzoyl-3',5'-di-O-benzoyl-5-iodo-2'-deoxy-2'-fluoroarabinouridine (2b) with unactivated Csp(3) fluoroalkylzinc bromides. This paper demonstrates the first synthesis of six 5-fluoroalkyl-2'-deoxypyrimidine nucleoside derivatives with three to five methylene chain lengths (5). Furthermore, this methodology has been extended to create a series of 13 5-alkyl-substituted nucleosides, including the target nucleosides 5 and 5-silyloxypropyl and 5-cyanobutyl derivatives.
Figures


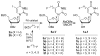

References
-
-
For examples see: Galmarini C, Mackey J, Dumontet C. Lancet Oncol. 2002;3:415–24.Balzarini J, McGuigan C. J Antimicrob Chemother. 2002;50:5–9.Gumina G, Song G, Chu C. FEMS Microbiol Lett. 2001;202:9–15.Jordheim LP, Galmarini CM, Dumontet C. Recent Patents Anticancer Drug Discov. 2006;1:163–70.
-
-
- Kumar R. Curr Med Chem. 2004;11:2749–66. and all references therein. - PubMed
-
-
Note that HSV1-tk denotes the gene and HSV1-TK denotes the protein (i.e. the gene product)
-
-
- Furman PA, McGuirt PV, Keller PM, Fyfe JA, Elion GB. Virology. 1980;102:420–30. - PubMed
- Freeman SM. Adv Exp Med Biol. 2000;465:411–22. - PubMed
- Mullen CA. Pharmacol Ther. 1994;63:199–207. - PubMed
- Wei MX, Bougnoux P, Sacre-Salem B, Peyrat MB, Lhuillery C, Salzmann JL, Klatzmann D. Cancer Res. 1998;58:3529–32. - PubMed
-
-
CAUTION: 18F is a radioactive material (radioactive half-life (t1/2) = 110 min, β+ emission = 511 keV) and should only be used by trained research personnel with the appropriate safety precautions.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

