A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day
- PMID: 18522516
- PMCID: PMC3683860
- DOI: 10.1101/sqb.2007.72.072
A circadian clock in Neurospora: how genes and proteins cooperate to produce a sustained, entrainable, and compensated biological oscillator with a period of about a day
Abstract
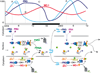
Neurospora has proven to be a tractable model system for understanding the molecular bases of circadian rhythms in eukaryotes. At the core of the circadian oscillatory system is a negative feedback loop in which two transcription factors, WC-1 and WC-2, act together to drive expression of the frq gene. WC-2 enters the promoter region of frq coincident with increases in frq expression and then exits when the cycle of transcription is over, whereas WC-1 can always be found there. FRQ promotes the phosphorylation of the WCs, thereby decreasing their activity, and phosphorylation of FRQ then leads to its turnover, allowing the cycle to reinitiate. By understanding the action of light and temperature on frq and FRQ expression, the molecular basis of circadian entrainment to environmental light and temperature cues can be understood, and recently a specific role for casein kinase 2 has been found in the mechanism underlying circadian temperature-compensation. These data promise molecular explanations for all of the canonical circadian properties of this model system, providing biochemical answers and regulatory logic that may be extended to more complex eukaryotes including humans.
Figures






References
-
- Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian CLOCK disrupts circadian rhythms and transcription of period and timeless . Cell. 1998;93:805. - PubMed
-
- Aronson B, Johnson K, Loros JJ, Dunlap JC. Negative feedback defining a circadian clock: Autoregulation in the clock gene frequency . Science. 1994b;263:1578. - PubMed
-
- Ballario P, Macino G. White collar proteins: PASsing the light signal in Neurospora crassa . Trends Microbiol. 1997;5:458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
