Mammary carcinoma behavior is programmed in the precancer stem cell
- PMID: 18522749
- PMCID: PMC2481504
- DOI: 10.1186/bcr2104
Mammary carcinoma behavior is programmed in the precancer stem cell
Abstract
Introduction: The 'MINO' (mammary intraepithelial neoplasia outgrowth) mouse model of ductal carcinoma in situ (DCIS) consists of six lines with distinct morphologic phenotypes and behavior, each meeting experimentally defined criteria for 'precancer'. Specifically, these lines grow orthotopically in cleared mammary fat pads and consistently progress to an invasive phenotype that is capable of ectopic growth. Transition to carcinoma has a consistent latency for each line, and three of the lines also exhibit pulmonary metastatic potential.
Methods: Gland cleared orthotopic transplanted precancer MINO tissues were analyzed by bacterial artifical chromosome and oligo array comparative genomic hybridization, microsatellite PCR, and telomerase repeat amplification assay. MINO cells were dissociated and cultured in three dimensional culture and transplanted in syngeneic gland cleared mammary fat pads.
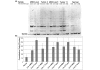
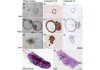
Results: Comparative genomic hybridization shows that the precancer and invasive tumors are genetically stable, with low level changes including whole chromosome gains in some lines. No changes are associated with progression, although spontaneous focal amplifications and deletions were detected occasionally. Microsatellite analysis shows a low frequency of alterations that are predominantly permanent within a MINO line. Telomerase activity is increased in both the MINO and the derived tumors when compared with normal mouse mammary gland. Dissociation of the precancer lesion cells and three dimensional 'spheroid' culture of single cells reveals a bipotential for myoepithelial and luminal differentiation and the formation of unique three-dimensional 'MINOspheres'. These MINOspheres exhibit features that are intermediate between spheroids that are derived from normal and carcinoma cells. Transplantation of a single cell derived MINOsphere recapitulates the outgrowth of the precancer morphology and progression to carcinoma.
Conclusion: These data establish a precancer 'stem' cell that is capable of self-renewal and multilineage differentiation as the origin of invasive cancer. Within the context of this model, these cells have programmed potential for latency and metastasis that does not appear to require sequential genetic 'hits' for transformation.
Figures